Solid State :
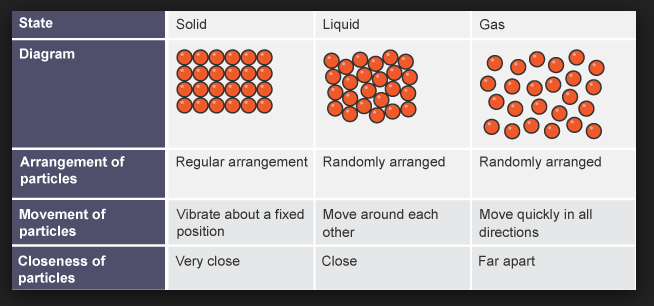
`=>` From our earlier studies we came to know that liquids and gases are called fluids as they have tendency to flow(molecules are free to move).
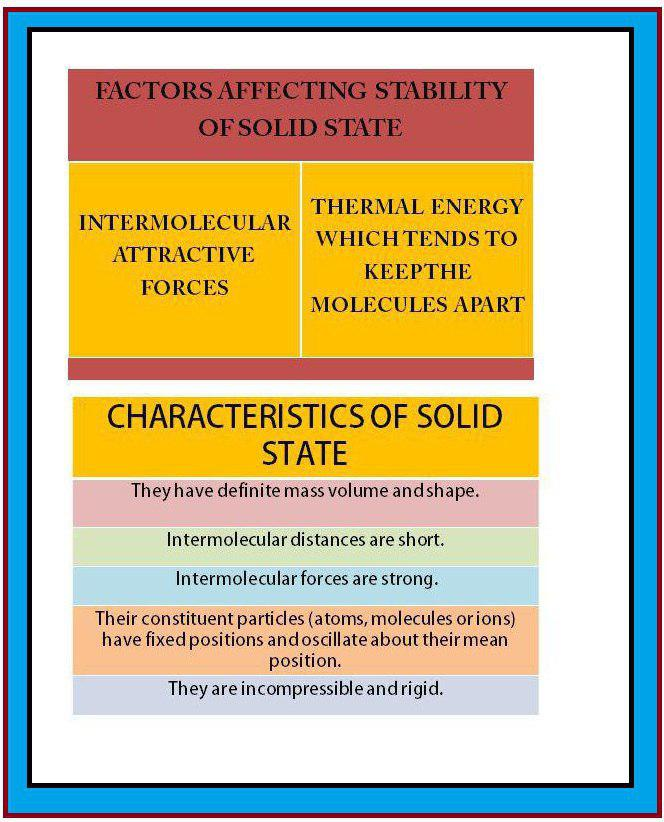
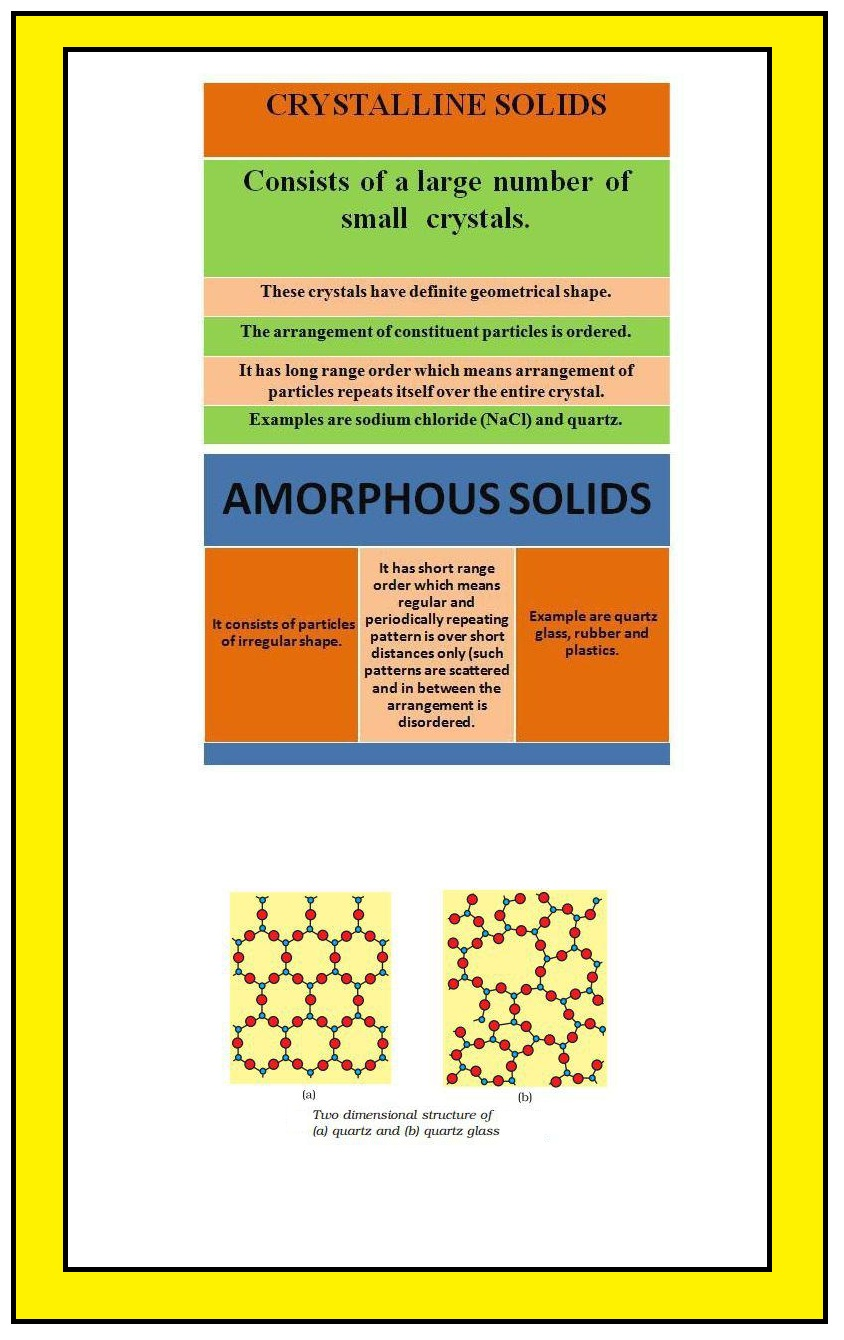
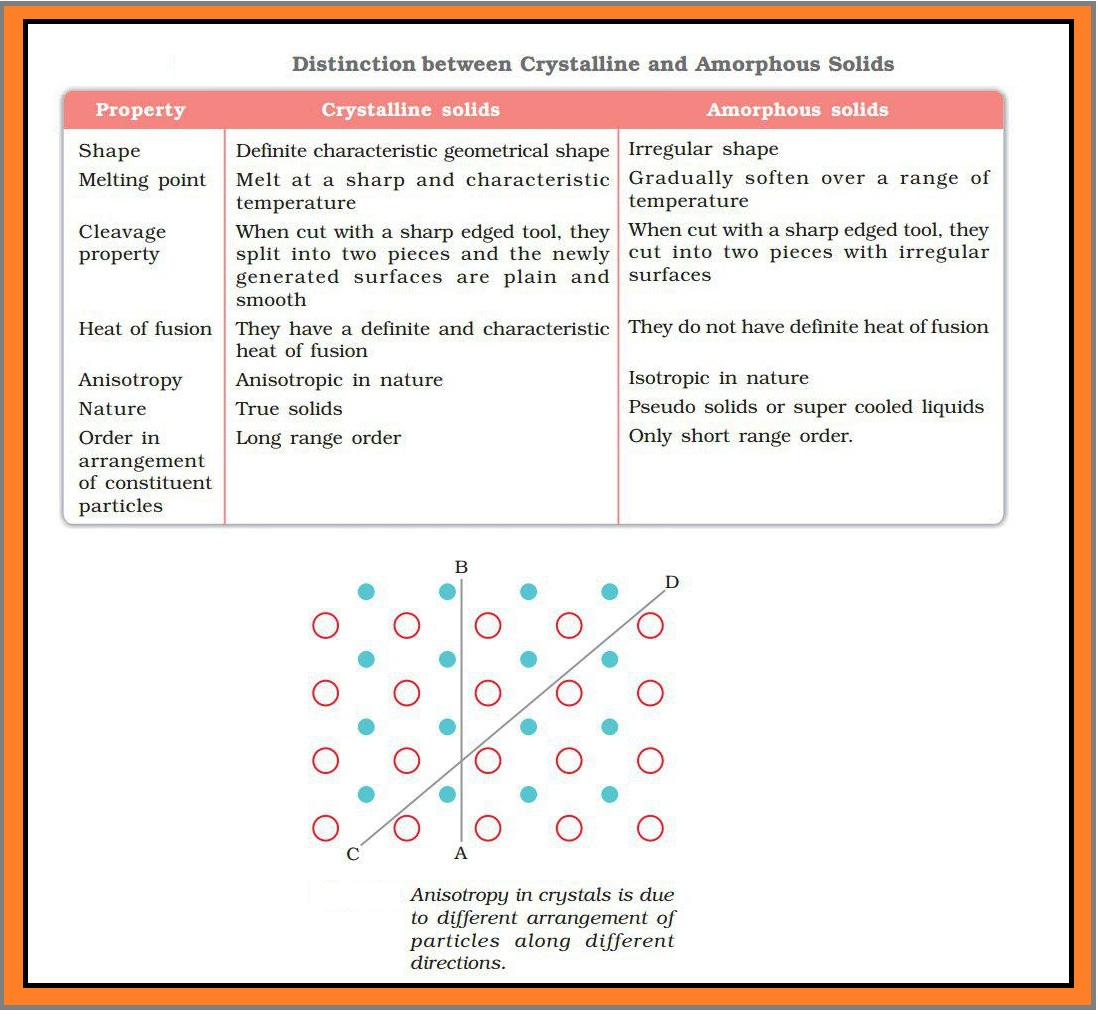
`=>` The constituent particles in solids have fixed positions and therefore can only oscillate about their mean positions. That's why solids are rigid.
`=>` The constituent particles in solids have fixed positions and therefore can only oscillate about their mean positions. That's why solids are rigid.