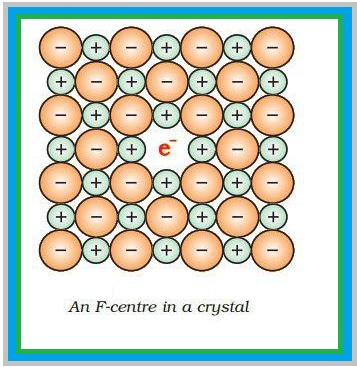
Types of Stoichiometric Defects :
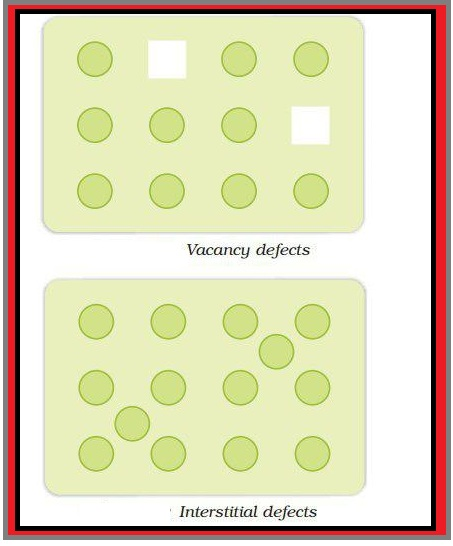
(i) Vacancy Defect : This is due to the vacancy present in the crystal. Due to this, density of crystal decreases. This defect can develop when a substance is heated.
(ii) Interstitial Defect : In this, some constituent particles occupy an interstitial site. Due to this defect, density of the substance increases.
Note : (i) Both these defects are shown by non-ionic solids.
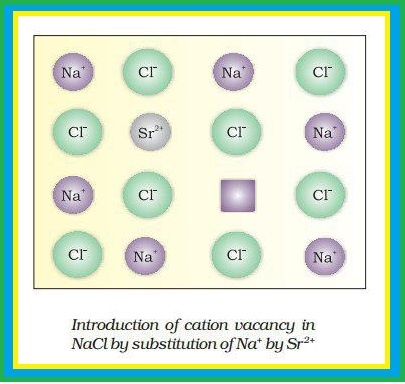
(ii) Ionic crystals always maintain electrical neutrality.
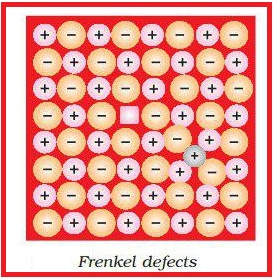
(iii) Vacancy and interstitial defects are shown as frankel and schottky defects.
(ii) Interstitial Defect : In this, some constituent particles occupy an interstitial site. Due to this defect, density of the substance increases.
Note : (i) Both these defects are shown by non-ionic solids.
(ii) Ionic crystals always maintain electrical neutrality.
(iii) Vacancy and interstitial defects are shown as frankel and schottky defects.