Law of Conservation of Mass :
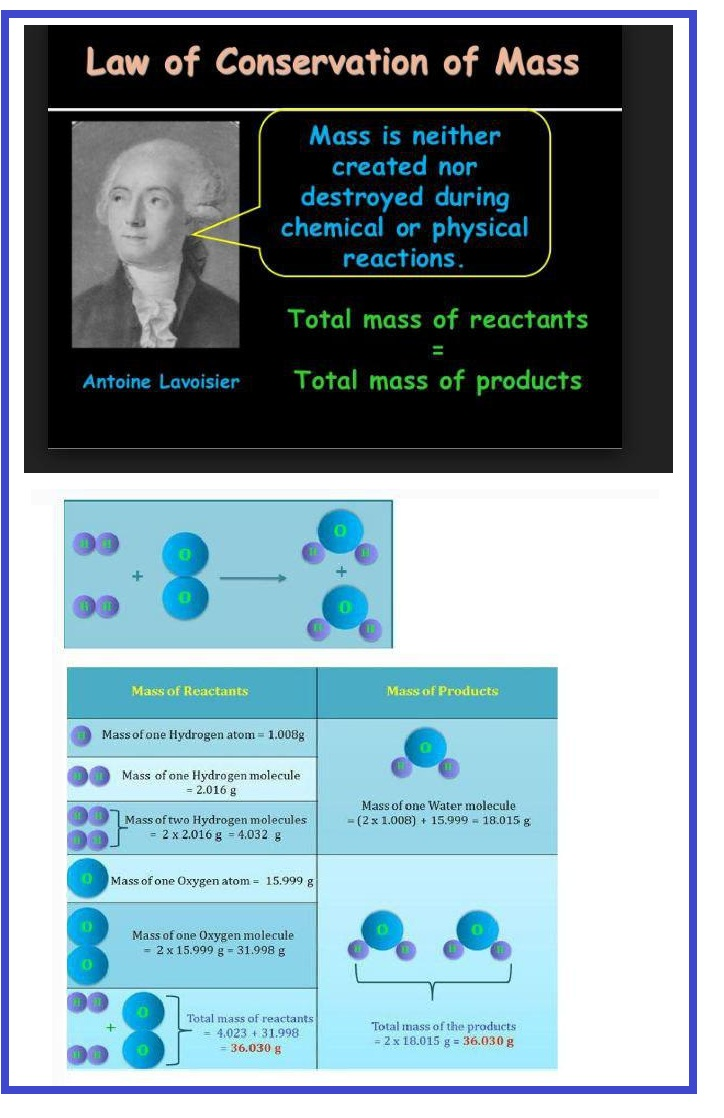
This law establishes the relationship between the masses of reactants and products during a chemical reaction. This law was postulated by A. Lavoisier in 1750.
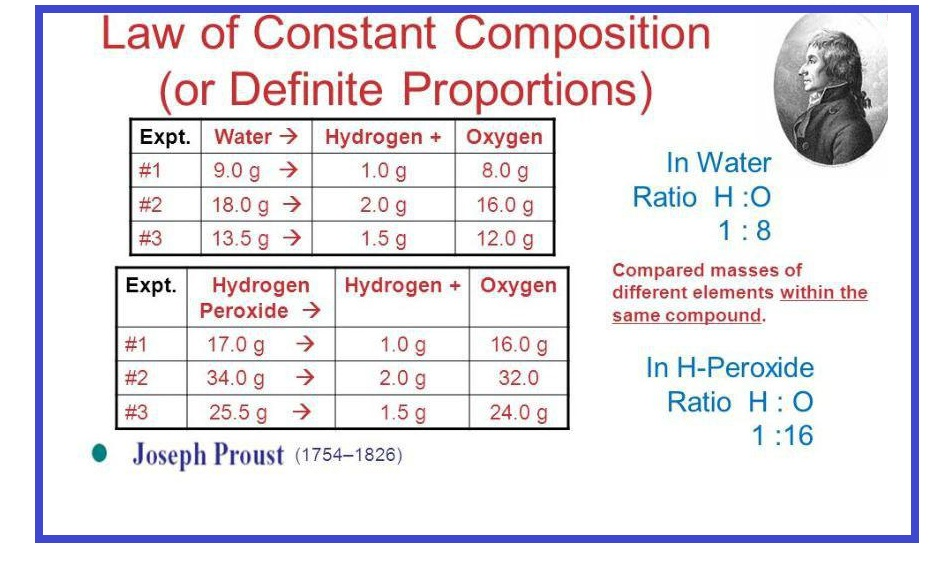
Statement : "Matter can neither be created nor destroyed during any physical or chemical change".
OR
"During any physical or chemical change, the total mass of the products is equal to the total mass of the reactants."
e. g. `underset(12 g) C+underset(32 g) O_2 → underset(44 g)(CO_2)`
Here, 12 g carbon combines with 32 g oxygen to give 44 g carbon dioxide. This law may be explained with the help of Landolt's experiments.
Statement : "Matter can neither be created nor destroyed during any physical or chemical change".
OR
"During any physical or chemical change, the total mass of the products is equal to the total mass of the reactants."
e. g. `underset(12 g) C+underset(32 g) O_2 → underset(44 g)(CO_2)`
Here, 12 g carbon combines with 32 g oxygen to give 44 g carbon dioxide. This law may be explained with the help of Landolt's experiments.