Moseley's Law
Frequency v of characteristic X -rays spectrum
`sqrtv = n(z - sigma)`
where, `a` and `sigma` are constants and screening constant for `K_alpha`
line, `sigma = 1`, Screening constant for `L_alpha` line, `sigma = 7.4`
`text(Bragg's Law)`
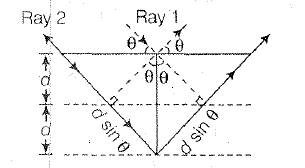
This law states that, when the X -ray is incident onto a crystal surface its angle of incidence `theta`, will reflect back with a same angle of scattering `theta`. And when the path difference `d` is equal to a whole number `n`, of wavelength `lamda`. A constructive interference will occur. Consider the diagram in which beam of an `X`-rays incident at an angle `theta` get diffracted as shown in figure. Clearly, path difference between ray `1` and ray `2` is `2d sin theta`.
For maxima of X-rays diffracted from the crystal. `2d sin theta = n lamda`
where, `n = 1 ,2,3, ... , d` is interatomic gap of the crystal
`sqrtv = n(z - sigma)`
where, `a` and `sigma` are constants and screening constant for `K_alpha`
line, `sigma = 1`, Screening constant for `L_alpha` line, `sigma = 7.4`
`text(Bragg's Law)`
This law states that, when the X -ray is incident onto a crystal surface its angle of incidence `theta`, will reflect back with a same angle of scattering `theta`. And when the path difference `d` is equal to a whole number `n`, of wavelength `lamda`. A constructive interference will occur. Consider the diagram in which beam of an `X`-rays incident at an angle `theta` get diffracted as shown in figure. Clearly, path difference between ray `1` and ray `2` is `2d sin theta`.
For maxima of X-rays diffracted from the crystal. `2d sin theta = n lamda`
where, `n = 1 ,2,3, ... , d` is interatomic gap of the crystal
