HYDROGEN
`=>` The discovery of hydrogen is credited to Henry Cavendish in 1766, although it had been isolated as early as 1671 by Robert Boyle.
`=>` Hydrogen is easily the most abundant element in the universe, although it constitutes only a very small percentage of the Earth's total mass.
`=>` Hydrogen is the first element in the periodic table and is the lightest element known.
`=>` It exists as a diatomic molecule `H_2` (dihydrogen).
`=>` It's name hydrogen was given by Lavoisier.
`=>` He prepared the gas by treating iron with dilute `H_2SO_4`.
`=>` Its atomic number is 1 and it has the electronic configuration `1s^1`.
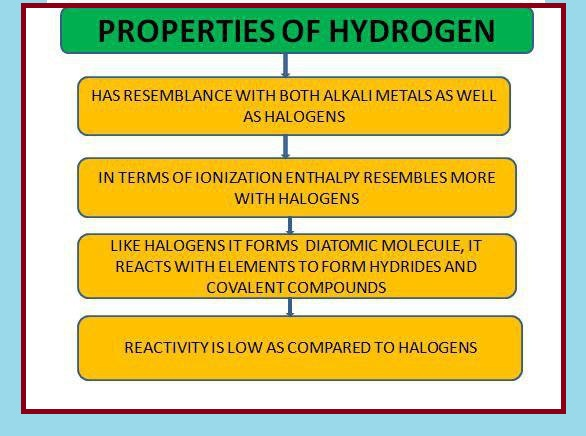
`=>` It resembles both alkali metals and halogens. e.g. it may donate one electron forming `H^(-1)` like alkali metals or may accept one electron forming `H^(-)` like halogens. But some properties of hydrogen are different with respect to these properties, therefore it is unique in behaviour so it is placed separately in the periodic table.
`=>` Jupiter and Saturn planets consist mainly of hydrogen.
`=>` Hydrogen is easily the most abundant element in the universe, although it constitutes only a very small percentage of the Earth's total mass.
`=>` Hydrogen is the first element in the periodic table and is the lightest element known.
`=>` It exists as a diatomic molecule `H_2` (dihydrogen).
`=>` It's name hydrogen was given by Lavoisier.
`=>` He prepared the gas by treating iron with dilute `H_2SO_4`.
`=>` Its atomic number is 1 and it has the electronic configuration `1s^1`.
`=>` It resembles both alkali metals and halogens. e.g. it may donate one electron forming `H^(-1)` like alkali metals or may accept one electron forming `H^(-)` like halogens. But some properties of hydrogen are different with respect to these properties, therefore it is unique in behaviour so it is placed separately in the periodic table.
`=>` Jupiter and Saturn planets consist mainly of hydrogen.