`=>` Oxygen dissolves in water to a small extent. This dissolved oxygen sustains all aquatic life.
`=>` On the other hand, hydrogen chloride gas (`HCl`) is highly soluble in water.
`=>` Solubility of gases in liquids is greatly affected by pressure and temperature.
`=>` The solubility of gases increase with increase of pressure.
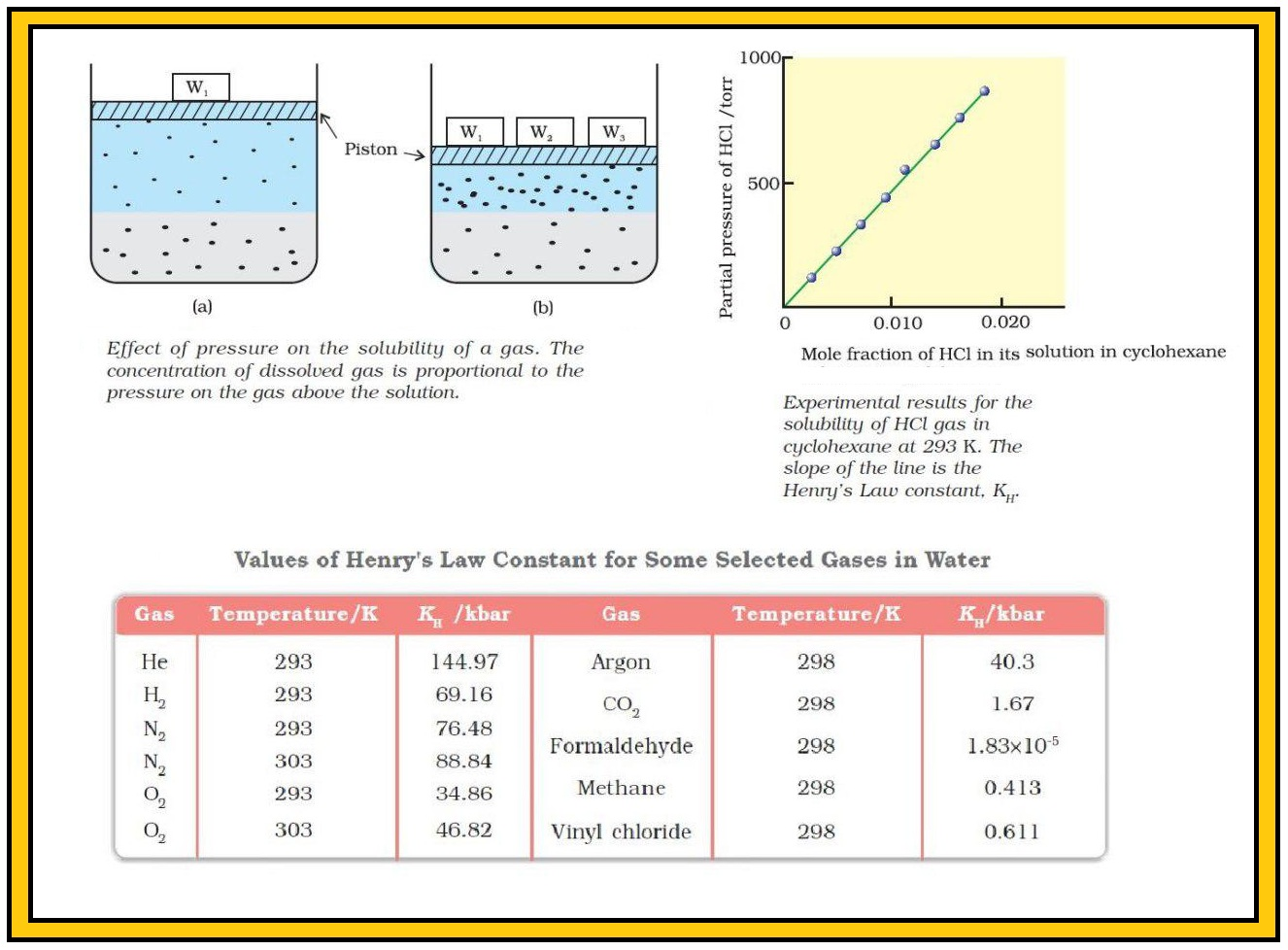
`color{green}("Explanation") :` For solution of gases in a solvent, consider a system as shown above. The lower part is solution and the upper part is gaseous system at pressure `p` and temperature `T`. In a state of dynamic equilibrium, i.e., under these conditions rate of gaseous particles entering and leaving the solution phase is the same. Now increase the pressure by compressing the gas to a smaller volume. This will increase the number of gaseous particles per unit volume over the solution and also the rate at which the gaseous particles are striking the surface of solution to enter it. So, the solubility of the gas will increase until a new equilibrium is reached resulting in an increase in the pressure of a gas above the solution and thus its solubility increases.
`color{green}("Henry's Law") :`
`=>` Henry gave a quantitative relation between pressure and solubility of a gas in a solvent which is known as Henry’s law.
`color{green}("Statement") :` At a constant temperature, the solubility of a gas in a liquid is directly proportional to the pressure of the gas.
`=>` Dalton, at the same time, also concluded independently that the solubility of a gas in a liquid solution is a function of partial pressure of the gas.
`=>` If mole fraction of a gas in the solution is used as a measure of its solubility, then mole fraction of gas in the solution is proportional to the partial pressure of the gas over the solution.
`=>` The most commonly used form of Henry’s law states that “the partial pressure of the gas in vapour phase (`p`) is proportional to the mole fraction of the gas (`x`) in the solution” and is expressed as :
`color{green}(p = K_H x)` ............2
Here `color{geen}(K_H)` is the Henry’s law constant.
`=>` A graph between partial pressure of the gas versus mole fraction of the gas in solution, then we get a plot of as shown above.
`=>` Different gases have different `K_H` values at the same temperature (Table). This shows that `K_H` is a function of the nature of the gas.
`=>` Higher the value of `K_H` at a given pressure, the lower is the solubility of the gas in the liquid.
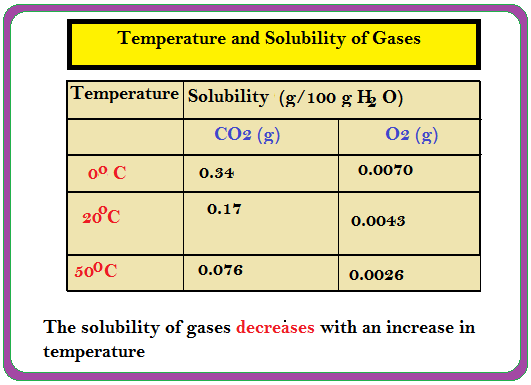
`=>` `K_H` values for both `N_2` and `O_2` increase with increase of temperature showing that the solubility of gases increases with decrease of temperature (Table).
`=>` Due to this reason that aquatic species are more comfortable in cold waters rather than in warm waters.
`=>` Oxygen dissolves in water to a small extent. This dissolved oxygen sustains all aquatic life.
`=>` On the other hand, hydrogen chloride gas (`HCl`) is highly soluble in water.
`=>` Solubility of gases in liquids is greatly affected by pressure and temperature.
`=>` The solubility of gases increase with increase of pressure.
`color{green}("Explanation") :` For solution of gases in a solvent, consider a system as shown above. The lower part is solution and the upper part is gaseous system at pressure `p` and temperature `T`. In a state of dynamic equilibrium, i.e., under these conditions rate of gaseous particles entering and leaving the solution phase is the same. Now increase the pressure by compressing the gas to a smaller volume. This will increase the number of gaseous particles per unit volume over the solution and also the rate at which the gaseous particles are striking the surface of solution to enter it. So, the solubility of the gas will increase until a new equilibrium is reached resulting in an increase in the pressure of a gas above the solution and thus its solubility increases.
`color{green}("Henry's Law") :`
`=>` Henry gave a quantitative relation between pressure and solubility of a gas in a solvent which is known as Henry’s law.
`color{green}("Statement") :` At a constant temperature, the solubility of a gas in a liquid is directly proportional to the pressure of the gas.
`=>` Dalton, at the same time, also concluded independently that the solubility of a gas in a liquid solution is a function of partial pressure of the gas.
`=>` If mole fraction of a gas in the solution is used as a measure of its solubility, then mole fraction of gas in the solution is proportional to the partial pressure of the gas over the solution.
`=>` The most commonly used form of Henry’s law states that “the partial pressure of the gas in vapour phase (`p`) is proportional to the mole fraction of the gas (`x`) in the solution” and is expressed as :
`color{green}(p = K_H x)` ............2
Here `color{geen}(K_H)` is the Henry’s law constant.
`=>` A graph between partial pressure of the gas versus mole fraction of the gas in solution, then we get a plot of as shown above.
`=>` Different gases have different `K_H` values at the same temperature (Table). This shows that `K_H` is a function of the nature of the gas.
`=>` Higher the value of `K_H` at a given pressure, the lower is the solubility of the gas in the liquid.
`=>` `K_H` values for both `N_2` and `O_2` increase with increase of temperature showing that the solubility of gases increases with decrease of temperature (Table).
`=>` Due to this reason that aquatic species are more comfortable in cold waters rather than in warm waters.