`=>` The vapour pressure of a liquid increases with increase of temperature.
`=>` It boils at the temperature at which its vapour pressure is equal to the atmospheric pressure.
`=>` Example, water boils at `373.15 K` (`100°C`) because at this temperature the vapour pressure of water is `1.013` bar (`1` atmosphere).
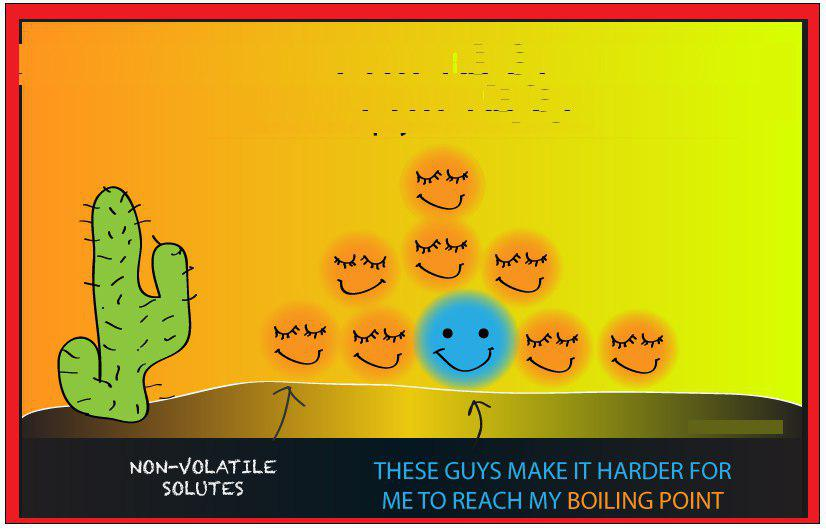
`=>` Vapour pressure of the solvent decreases in the presence of non-volatile solute.
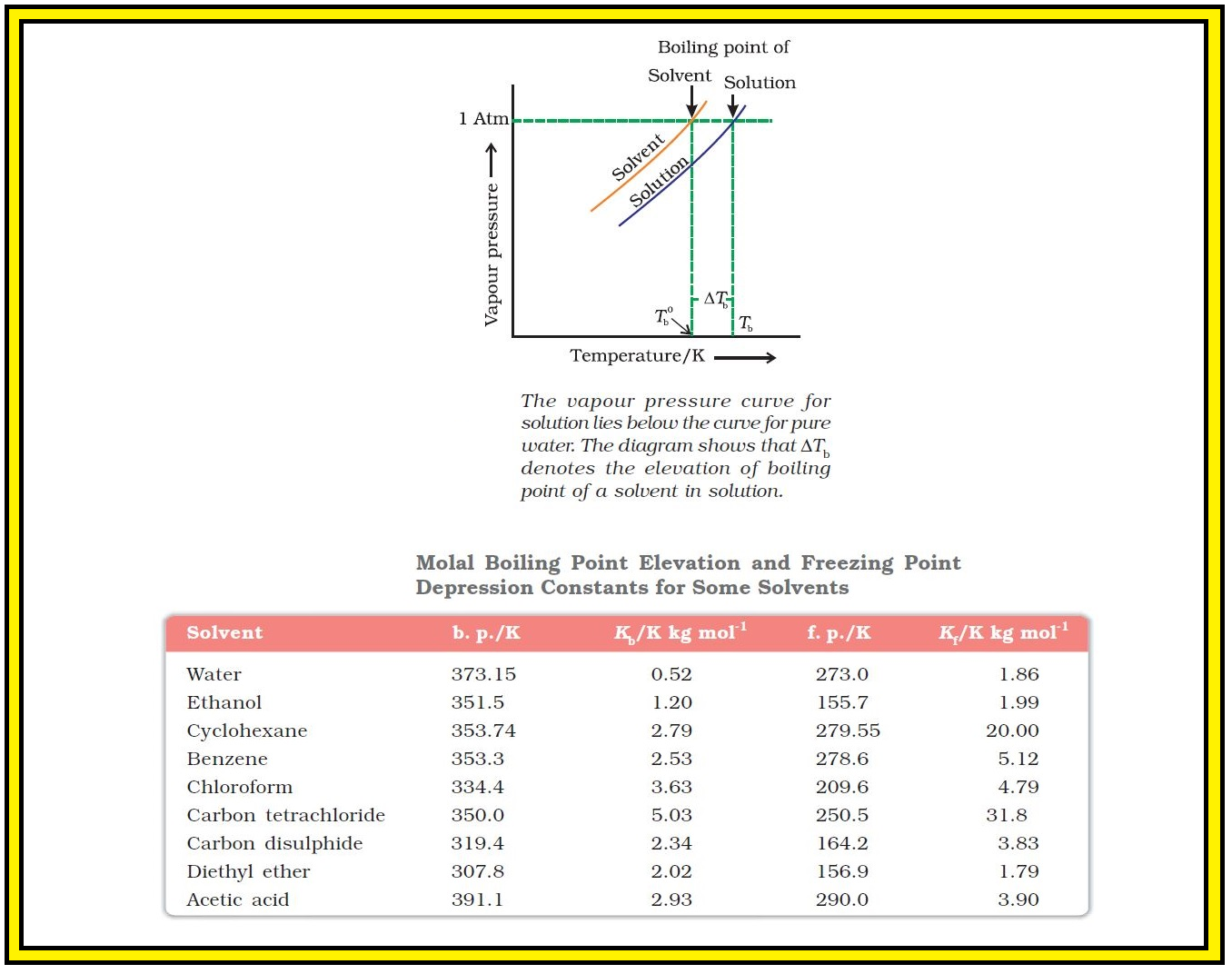
`=>` Variation of vapour pressure of the pure solvent and solution as a function of temperature is shown.
`=>` Example, the vapour pressure of an aqueous solution of sucrose is less than `1.013` bar at `373.15 K`. In order to make this solution boil, its vapour pressure must be increased to `1.013` bar by raising the temperature above the boiling temperature of the pure solvent (water). Thus, the boiling point of a solution is always higher than that of the boiling point of the pure solvent in which the solution is prepared as shown in Fig. 2.7.
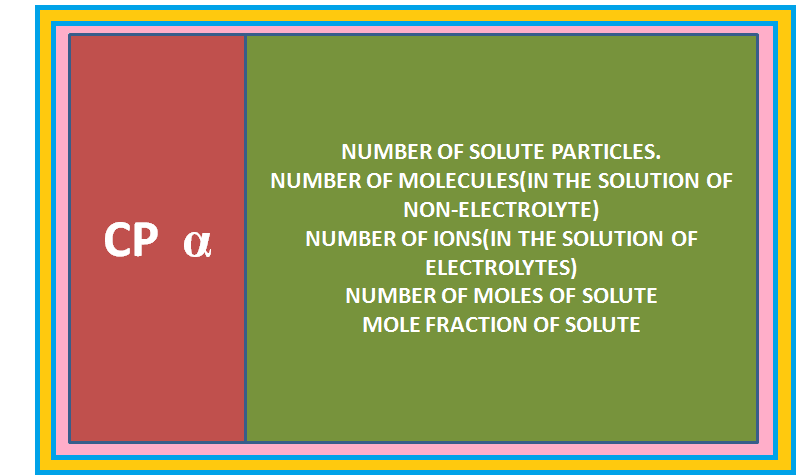
`=>` The elevation of boiling point also depends on the number of solute molecules rather than their nature.
Let `T_b^0` be the boiling point of pure solvent and `T_b` be the boiling point of solution. The increase in the boiling point `DeltaT_b = T_b - T_b^0` is known as elevation of boiling point.
Experimentally, for dilute solutions the elevation of boiling point `(ΔT_b)` is directly proportional to the molal concentration of the solute in a solution.
Thus, `color{red}(DeltaT_b prop m) ` .................(8)
or `color{red}(DeltaT_b = K_b m)` ............(9)
Here, the constant of proportionality `K_b` is called Boiling Point Elevation Constant or Molal Elevation Constant (Ebullioscopic Constant).
● The unit of `K_b` is `K kg mol^(-1)`.
● Values of `K_b` for some common solvents are given in Table
`=>` If `w_2` gram of solute of molar mass `M_2` is dissolved in `w_1` gram of solvent, then molality, `m` of the solution is given by the expression :
`color{red}(m = ( w_2/M_2)/(w_1/1000) = ( 1000xx w_2)/(M_2 xx w_1))` .....................(10).
Substituting the value of molality in equation (2.30) we get `color{red}(DeltaT_b = (K_b xx 1000 xx w_2)/(M_2 xx w_1))` .............(11).
`color{red}(M_2 = (1000 xx w_2 xx K_b)/(DeltaT_b xx w_1))` ............(12).
Thus, for determining `M_2`, molar mass of the solute, known mass of solute in a known mass of the solvent is taken and `ΔT_b` is determined experimentally for a known solvent whose `K_b` value is known.
`=>` Value of `K_b` is given as :
`color{red}(K_b = (R xx M_1 xx T_b^2)/(1000 xx Delta_text(vap) H))` ..............(13).
Here the symbols `R` and `M_1` stand for the gas constant and molar mass of the solvent, respectively and `T_b` denote the boiling point of the pure solvent respectively in kelvin.
Further, `Δ_text(vap)H` represent the enthalpy of vapourisation of the solvent, respectively.
`=>` The vapour pressure of a liquid increases with increase of temperature.
`=>` It boils at the temperature at which its vapour pressure is equal to the atmospheric pressure.
`=>` Example, water boils at `373.15 K` (`100°C`) because at this temperature the vapour pressure of water is `1.013` bar (`1` atmosphere).
`=>` Vapour pressure of the solvent decreases in the presence of non-volatile solute.
`=>` Variation of vapour pressure of the pure solvent and solution as a function of temperature is shown.
`=>` Example, the vapour pressure of an aqueous solution of sucrose is less than `1.013` bar at `373.15 K`. In order to make this solution boil, its vapour pressure must be increased to `1.013` bar by raising the temperature above the boiling temperature of the pure solvent (water). Thus, the boiling point of a solution is always higher than that of the boiling point of the pure solvent in which the solution is prepared as shown in Fig. 2.7.
`=>` The elevation of boiling point also depends on the number of solute molecules rather than their nature.
Let `T_b^0` be the boiling point of pure solvent and `T_b` be the boiling point of solution. The increase in the boiling point `DeltaT_b = T_b - T_b^0` is known as elevation of boiling point.
Experimentally, for dilute solutions the elevation of boiling point `(ΔT_b)` is directly proportional to the molal concentration of the solute in a solution.
Thus, `color{red}(DeltaT_b prop m) ` .................(8)
or `color{red}(DeltaT_b = K_b m)` ............(9)
Here, the constant of proportionality `K_b` is called Boiling Point Elevation Constant or Molal Elevation Constant (Ebullioscopic Constant).
● The unit of `K_b` is `K kg mol^(-1)`.
● Values of `K_b` for some common solvents are given in Table
`=>` If `w_2` gram of solute of molar mass `M_2` is dissolved in `w_1` gram of solvent, then molality, `m` of the solution is given by the expression :
`color{red}(m = ( w_2/M_2)/(w_1/1000) = ( 1000xx w_2)/(M_2 xx w_1))` .....................(10).
Substituting the value of molality in equation (2.30) we get `color{red}(DeltaT_b = (K_b xx 1000 xx w_2)/(M_2 xx w_1))` .............(11).
`color{red}(M_2 = (1000 xx w_2 xx K_b)/(DeltaT_b xx w_1))` ............(12).
Thus, for determining `M_2`, molar mass of the solute, known mass of solute in a known mass of the solvent is taken and `ΔT_b` is determined experimentally for a known solvent whose `K_b` value is known.
`=>` Value of `K_b` is given as :
`color{red}(K_b = (R xx M_1 xx T_b^2)/(1000 xx Delta_text(vap) H))` ..............(13).
Here the symbols `R` and `M_1` stand for the gas constant and molar mass of the solvent, respectively and `T_b` denote the boiling point of the pure solvent respectively in kelvin.
Further, `Δ_text(vap)H` represent the enthalpy of vapourisation of the solvent, respectively.