The potential of individual half-cell cannot be measured. We can measure only the difference between the two half-cell potentials that gives the emf of the cell. It can be measured by using some electrode as the reference electrode. If we arbitrarily choose the potential of one electrode (half- cell) then that of the other can be determined with respect to this.
`color{green}("Standard Hydrogen Electrode :")`
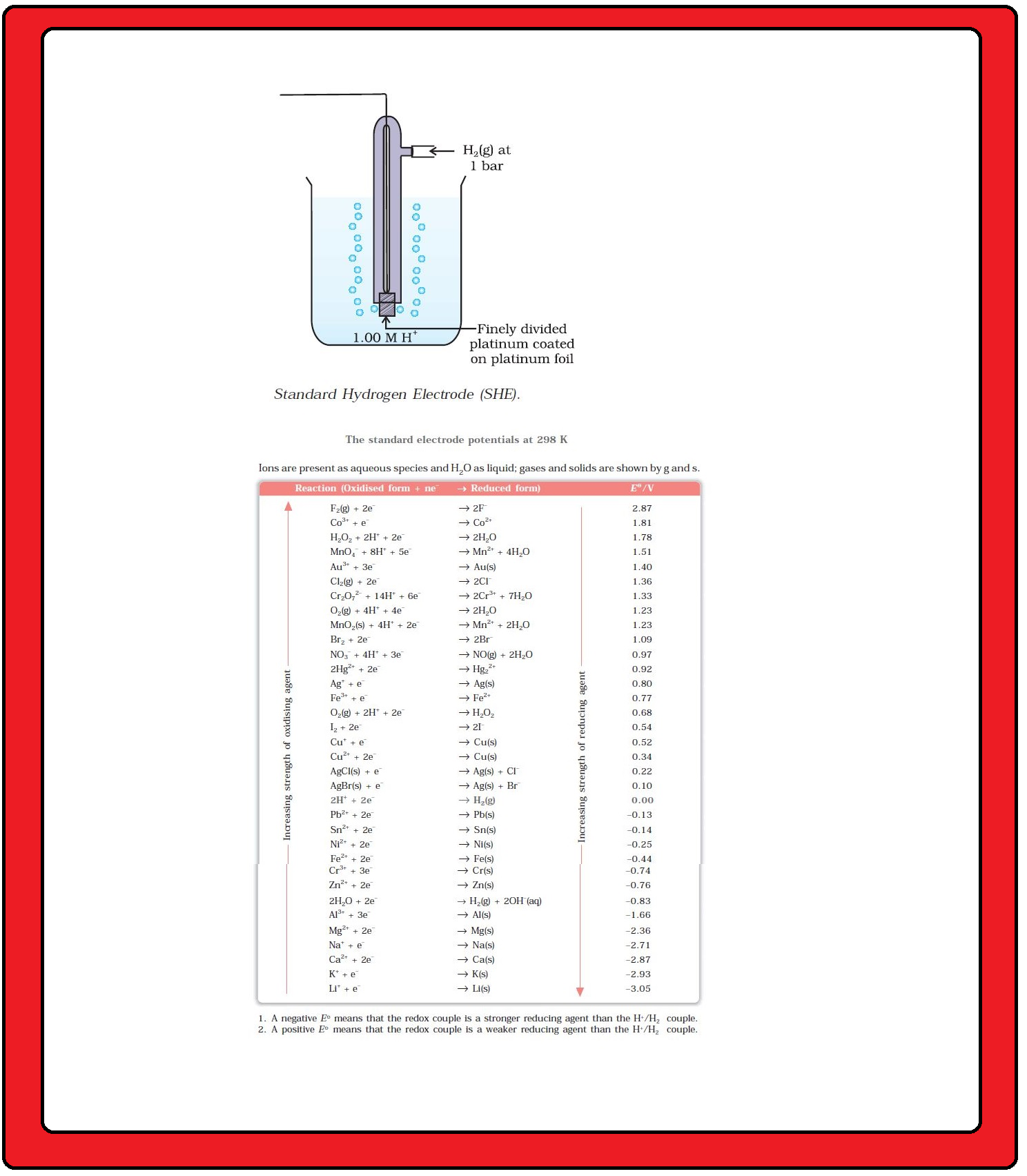
According to convention, a half-cell called standard hydrogen electrode represented by
`color{red}(Pt(s) | H_2(g) | H^(+) (aq))`,
is assigned a zero potential at all temperatures corresponding to the reaction `color{red}(H^(+) (aq) +e^(-) → 1/2 H_2 (g))`.
`=>` The standard hydrogen electrode consists of a platinum electrode coated with platinum black.
`=>` The electrode is dipped in an acidic solution and pure hydrogen gas is bubbled through it.
`=>` The concentration of both the reduced and oxidised forms of hydrogen is maintained at unity . This implies that the pressure of hydrogen gas is one bar and the concentration of hydrogen ion in the solution is one molar.
At 298 K the emf of the cell, standard hydrogen electrode | | second half-cell constructed by taking standard hydrogen
electrode as anode (reference half-cell) and the other half-cell as cathode, gives the reduction potential of the other half-cell. If
the concentrations of the oxidised and the reduced forms of the species in the right hand half-cell are unity, then the cell potential is equal to standard electrode potential `color{red}(E_R^(⊖) - E_L^(⊖))` As `color{red}(E_L^(⊖))` for standard hydrogen electrode is zero.
`color{red}(E^(⊖) = E_R^(⊖) - 0 = E_R^(⊖))`
`color{green}("The measured emf of the cell")` : `color{red}(Pt(s) | H_2 (g , 1 text(bar) | H^(+) (aq , 1 M) | | Cu^(2+) (aq , 1 M) | Cu)`
is `0.34 V` and it is also the value for the standard electrode potential of the half-cell corresponding to the reaction :
`color{red}(Cu^(2+) (aq , 1 M) +2 e^(-) → Cu(s))`.
`color{green}("Similarly, the measured emf of the cell :")` `color{red}(Pt(s) | H_2 (g , 1 text(bar) ) | H^(+) (aq , 1M) | | Zn^(2+) (aq , 1 M) | Zn)` is -0.76 V corresponding to the standard electrode potential of the half-cell reaction :
`color{red}(Zn^(2+) (aq , 1M) +2e^(-) → Zn(s))`.
`=>` The positive value of the standard electrode potential in the first case indicates that `Cu^(2+)` ions get reduced more easily than `H^+` ions. The reverse process cannot occur, that is, hydrogen ions cannot oxidise `Cu` (or alternatively we can say that hydrogen gas can reduce copper ion) under the standard conditions described above. Thus, `Cu` does not dissolve in `HCl`. In nitric acid it is oxidised by nitrate ion and not by hydrogen ion.
`=>` The negative value of the standard electrode potential in the second case indicates that hydrogen ions can oxidise zinc (or zinc can reduce hydrogen ions).
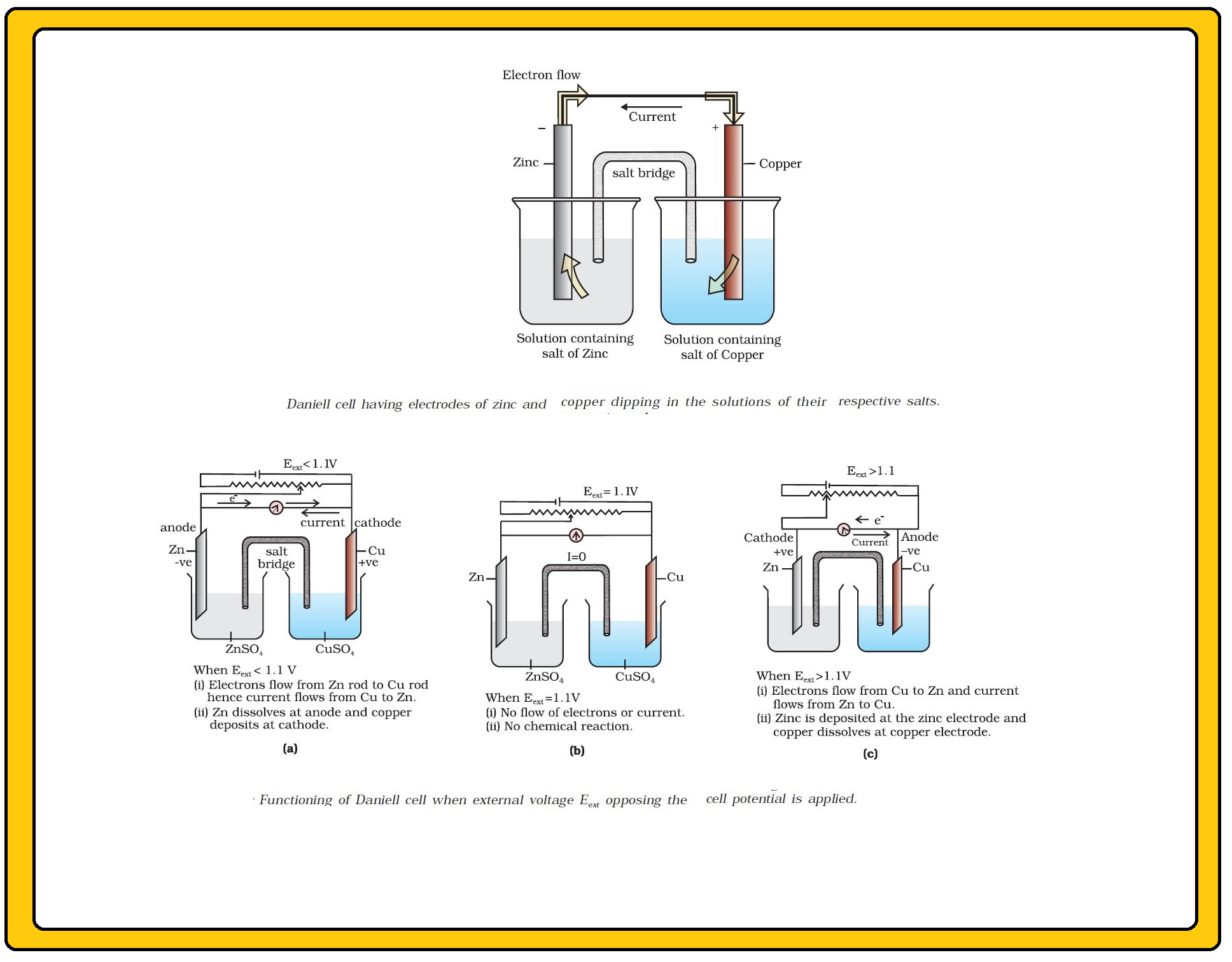
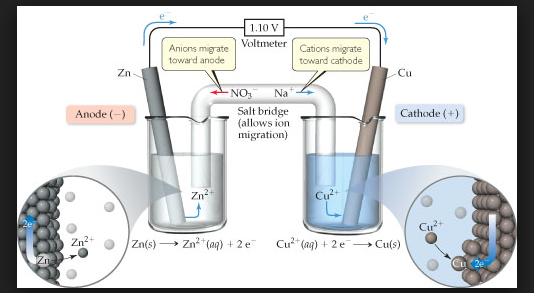
In view of this convention, the half reaction for the Daniell cell in Fig can be written as :
`color{green}("Left electrode")` : `color{red}(Zn (s) → Zn^(2+) (aq , 1M) +2e^(-))`.
`color{green}("Right electrode")` : `color{red}(Cu^(2+) (aq , 1M) +2 e^(-) → Cu(s))`.
The overall reaction of the cell is the sum of above two reactions and we obtain the equation :
`color{red}(Zn(s) +Cu^(2+) (aq) → Zn^(2+) (aq) + Cu(s))`
Emf of the cell `color{red}( = E_text(cell)^0 = E_R^0 - E_L^0)`
`color{red}( = 0.34V - (-0.76) V = 1.10 V)`
`color{green}("Note") :` Sometimes metals like platinum or gold are used as inert electrodes. They do not participate in the reaction but provide their surface for oxidation or reduction reactions and for the conduction of electrons.
For example, `Pt` is used in the following half-cells :
`color{green}("Hydrogen electrode")` : `color{red}(Pt(s) | H_2 (g) | H^(+) (aq))`.
`color{green}("With half-cell reaction")` : `color{red}(H^(+) (aq) + e^(-) → 1/2 H_2 (g))`.
`color{green}("Bromine electrode ")` : `color{red}(Pt(s) | Br_2(aq) | Br^(-) (aq))`.
`color{green}("With half-cell reaction")` : `color{red}(1/2 Br_2(aq) + e^(-) → Br^(-) (aq))`.
The standard electrode potentials are very important and we can extract a lot of useful information from them. The values of standard electrode potentials for some selected half-cell reduction reactions are given in Table.
`=>` If the standard electrode potential of an electrode is greater than zero then its reduced form is more stable compared to hydrogen gas.
`=>` If the standard electrode potential is negative then hydrogen gas is more stable than the reduced form of the species.
`=>` It can be seen that the standard electrode potential for fluorine is the highest in the Table indicating that fluorine gas `(F_2)` has the maximum tendency to get reduced to fluoride ions `(F^–)` and therefore fluorine gas is the strongest oxidising agent and fluoride ion is the weakest reducing agent.
`=>` Lithium has the lowest electrode potential indicating that lithium ion is the weakest oxidising agent while lithium metal is the most
powerful reducing agent in an aqueous solution.
`=>` It may be seen that as we go from top to bottom in Table 3.1 the standard electrode potential decreases and with this, decreases the oxidising power of the species on the left and increases the reducing power of the species on the right hand side of the reaction.
`=>` Electrochemical cells are extensively used for determining the pH of solutions, solubility product, equilibrium constant and other thermodynamic properties and for potentiometric titrations.
The potential of individual half-cell cannot be measured. We can measure only the difference between the two half-cell potentials that gives the emf of the cell. It can be measured by using some electrode as the reference electrode. If we arbitrarily choose the potential of one electrode (half- cell) then that of the other can be determined with respect to this.
`color{green}("Standard Hydrogen Electrode :")`
According to convention, a half-cell called standard hydrogen electrode represented by
`color{red}(Pt(s) | H_2(g) | H^(+) (aq))`,
is assigned a zero potential at all temperatures corresponding to the reaction `color{red}(H^(+) (aq) +e^(-) → 1/2 H_2 (g))`.
`=>` The standard hydrogen electrode consists of a platinum electrode coated with platinum black.
`=>` The electrode is dipped in an acidic solution and pure hydrogen gas is bubbled through it.
`=>` The concentration of both the reduced and oxidised forms of hydrogen is maintained at unity . This implies that the pressure of hydrogen gas is one bar and the concentration of hydrogen ion in the solution is one molar.
At 298 K the emf of the cell, standard hydrogen electrode | | second half-cell constructed by taking standard hydrogen
electrode as anode (reference half-cell) and the other half-cell as cathode, gives the reduction potential of the other half-cell. If
the concentrations of the oxidised and the reduced forms of the species in the right hand half-cell are unity, then the cell potential is equal to standard electrode potential `color{red}(E_R^(⊖) - E_L^(⊖))` As `color{red}(E_L^(⊖))` for standard hydrogen electrode is zero.
`color{red}(E^(⊖) = E_R^(⊖) - 0 = E_R^(⊖))`
`color{green}("The measured emf of the cell")` : `color{red}(Pt(s) | H_2 (g , 1 text(bar) | H^(+) (aq , 1 M) | | Cu^(2+) (aq , 1 M) | Cu)`
is `0.34 V` and it is also the value for the standard electrode potential of the half-cell corresponding to the reaction :
`color{red}(Cu^(2+) (aq , 1 M) +2 e^(-) → Cu(s))`.
`color{green}("Similarly, the measured emf of the cell :")` `color{red}(Pt(s) | H_2 (g , 1 text(bar) ) | H^(+) (aq , 1M) | | Zn^(2+) (aq , 1 M) | Zn)` is -0.76 V corresponding to the standard electrode potential of the half-cell reaction :
`color{red}(Zn^(2+) (aq , 1M) +2e^(-) → Zn(s))`.
`=>` The positive value of the standard electrode potential in the first case indicates that `Cu^(2+)` ions get reduced more easily than `H^+` ions. The reverse process cannot occur, that is, hydrogen ions cannot oxidise `Cu` (or alternatively we can say that hydrogen gas can reduce copper ion) under the standard conditions described above. Thus, `Cu` does not dissolve in `HCl`. In nitric acid it is oxidised by nitrate ion and not by hydrogen ion.
`=>` The negative value of the standard electrode potential in the second case indicates that hydrogen ions can oxidise zinc (or zinc can reduce hydrogen ions).
In view of this convention, the half reaction for the Daniell cell in Fig can be written as :
`color{green}("Left electrode")` : `color{red}(Zn (s) → Zn^(2+) (aq , 1M) +2e^(-))`.
`color{green}("Right electrode")` : `color{red}(Cu^(2+) (aq , 1M) +2 e^(-) → Cu(s))`.
The overall reaction of the cell is the sum of above two reactions and we obtain the equation :
`color{red}(Zn(s) +Cu^(2+) (aq) → Zn^(2+) (aq) + Cu(s))`
Emf of the cell `color{red}( = E_text(cell)^0 = E_R^0 - E_L^0)`
`color{red}( = 0.34V - (-0.76) V = 1.10 V)`
`color{green}("Note") :` Sometimes metals like platinum or gold are used as inert electrodes. They do not participate in the reaction but provide their surface for oxidation or reduction reactions and for the conduction of electrons.
For example, `Pt` is used in the following half-cells :
`color{green}("Hydrogen electrode")` : `color{red}(Pt(s) | H_2 (g) | H^(+) (aq))`.
`color{green}("With half-cell reaction")` : `color{red}(H^(+) (aq) + e^(-) → 1/2 H_2 (g))`.
`color{green}("Bromine electrode ")` : `color{red}(Pt(s) | Br_2(aq) | Br^(-) (aq))`.
`color{green}("With half-cell reaction")` : `color{red}(1/2 Br_2(aq) + e^(-) → Br^(-) (aq))`.
The standard electrode potentials are very important and we can extract a lot of useful information from them. The values of standard electrode potentials for some selected half-cell reduction reactions are given in Table.
`=>` If the standard electrode potential of an electrode is greater than zero then its reduced form is more stable compared to hydrogen gas.
`=>` If the standard electrode potential is negative then hydrogen gas is more stable than the reduced form of the species.
`=>` It can be seen that the standard electrode potential for fluorine is the highest in the Table indicating that fluorine gas `(F_2)` has the maximum tendency to get reduced to fluoride ions `(F^–)` and therefore fluorine gas is the strongest oxidising agent and fluoride ion is the weakest reducing agent.
`=>` Lithium has the lowest electrode potential indicating that lithium ion is the weakest oxidising agent while lithium metal is the most
powerful reducing agent in an aqueous solution.
`=>` It may be seen that as we go from top to bottom in Table 3.1 the standard electrode potential decreases and with this, decreases the oxidising power of the species on the left and increases the reducing power of the species on the right hand side of the reaction.
`=>` Electrochemical cells are extensively used for determining the pH of solutions, solubility product, equilibrium constant and other thermodynamic properties and for potentiometric titrations.