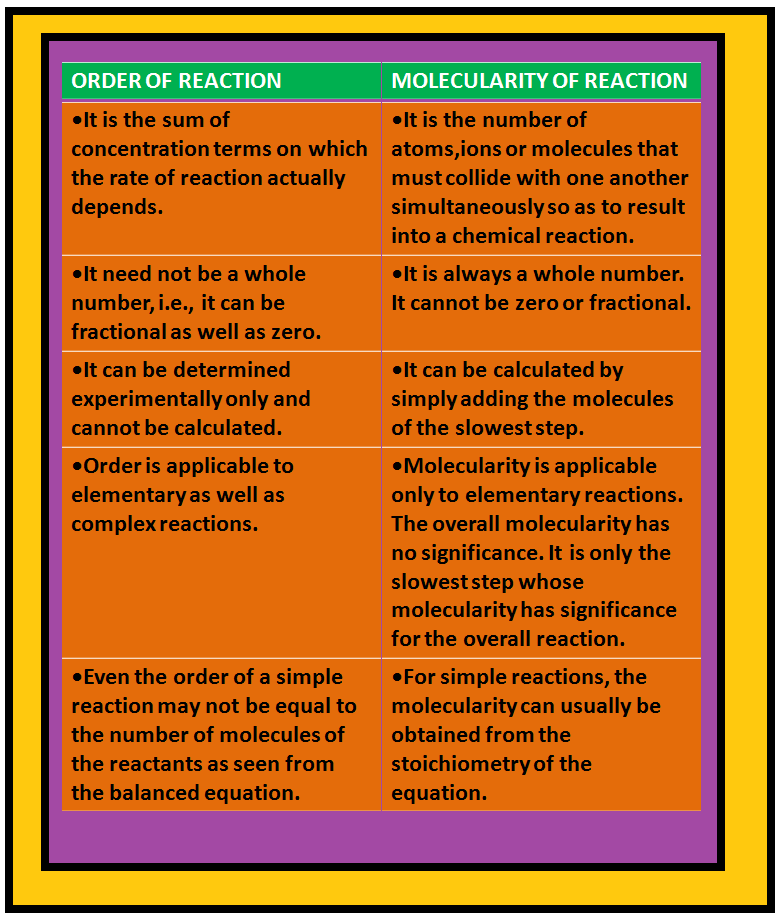
Rate Expression and Rate Constant :
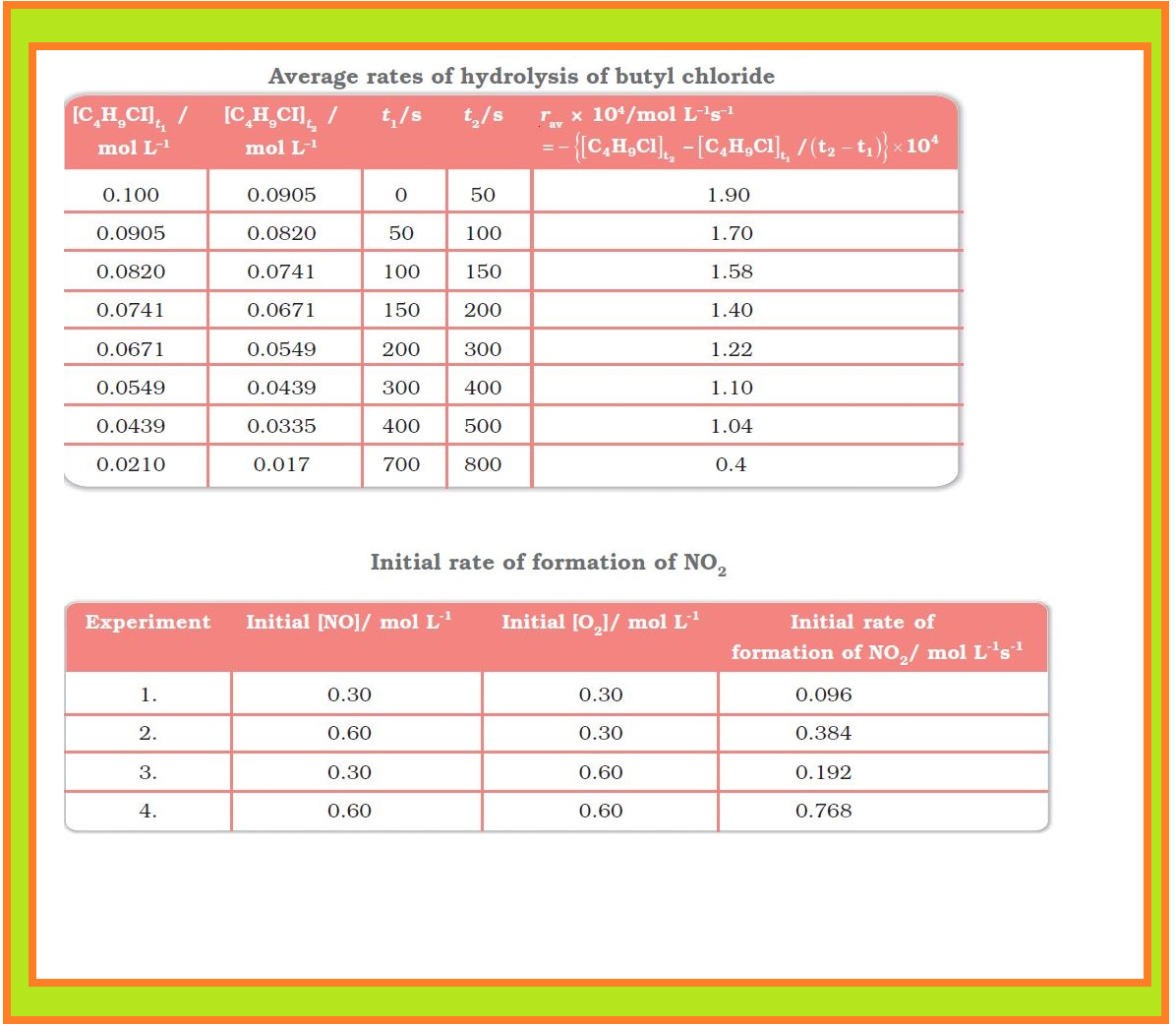
`=>` The results in Table clearly show that rate of a reaction decreases with the passage of time as the concentration of reactants decrease.
`=>` Conversely, rates generally increase when reactant concentrations increase. So, rate of a reaction depends upon the concentration of reactants.
`=>` Consider a general reaction `aA + bB → c C + d D`
where `a`, `b`, `c` and `d` are the stoichiometric coefficients of reactants and products. The rate expression for this reaction is
Rate `prop [A]^x [B]^y` ..................(1).
where exponents `x` and `y` may or may not be equal to the stoichiometric coefficients (`a` and `b`) of the reactants. Above equation
can also be written as
Rate = `k [A]^x [B]^y` ...............(2).
` - (d [R])/(dt) = k [A]^x [B]^y` ...............(3).
● This form of equation (3) is known as differential rate equation, where `k` is a proportionality constant called rate constant.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
Rate constant may be defined as the rate of the reaction when the molar concentration of each reactant is taken as unity. That is why the rate constant is also called specific reaction rate.
`color{green} ✍️ color{green} mathbf("Characteristics of rate constant")`:
•Greater is the value of the rate constant, faster is the reaction.
•Each reaction has a definite value of the rate constant at a particular temperature.
•The value of the rate constant for the same reaction changes with temperature.
•The value of the rate constant of a reaction does not depend upon the concentrations of the reactants.
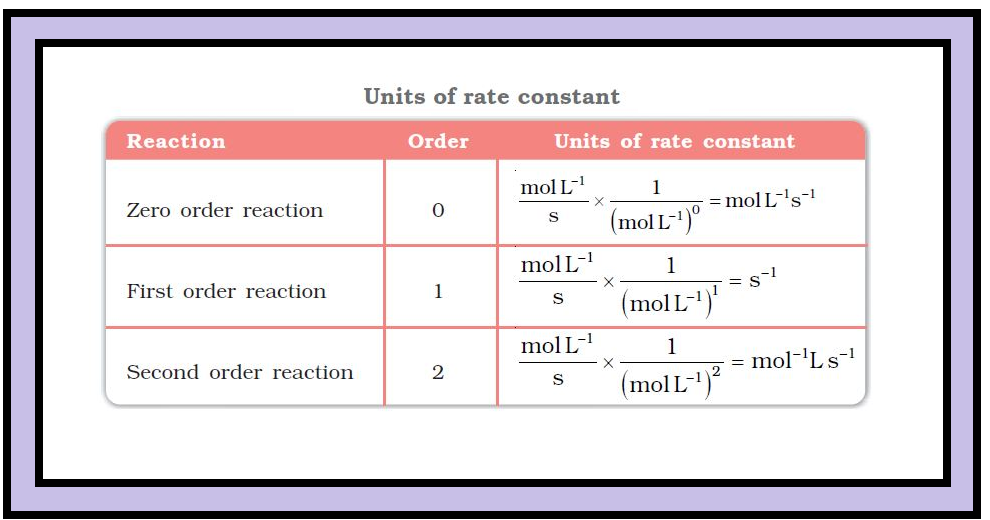
•The units of the rate constant depends upon the order of the reaction.
`text(Rate Law of Rate Expression) :` The equation which relates the rate of a reaction to concentration of reactants is called rate law or rate expression.
● Thus, rate law is the expression in which reaction rate is given in terms of molar concentration of reactants with each term raised to some power, which may or may not be same as the stoichiometric coefficient of the reacting species in a balanced chemical equation.
● For example :
`2NO(g) + O_2 (g) → 2NO_2 (g)`
We can measure the rate of this reaction as a function of initial concentrations either by keeping the concentration of one of the reactants constant and changing the concentration of the other reactant or by changing the concentration of both the reactants.
The following results are obtained:
`->` It is obvious, after looking at the results, that when the concentration of `NO` is doubled and that of `O_2` is kept constant then the initial rate increases by a factor of four from `0.096` to `0.384 mol L^(–1)s^(–1)`.
`->` This indicates that the rate depends upon the square of the concentration of `NO`.
`->` When concentration of `NO` is kept constant and concentration of `O_2` is doubled the rate also gets doubled indicating that rate depends on concentration of `O_2` to the first power.
`->` Hence, the rate equation for this reaction will be
Rate `= k[NO]_2[O_2]`
`->` The differential form of this rate expression is given as :
`- (d [R])/(dt) = k [NO]^2 [O_2]`
`->` Here, we observe that for this reaction in the rate equation derived from the experimental data, the exponents of the concentration terms are the same as their stoichiometric coefficients in the balanced chemical equation.
● Some other examples are given below :
Reaction Experimental rate expression
1. `CHCl_3+Cl_2 → C Cl_4+HCl` `Rate = k [CHCl_3] [Cl_2]^(1/2)`
2. `CH_3COOC_2H_5+H_2O → CH_3COOH +C_2H_5OH` `Rate = k [CH_3COOC_2H_5]^1 [H_2O]^0`
`->` In these reactions, the exponents of the concentration terms are not the same as their stoichiometric coefficients.
`=>` Thus, we can say that Rate law for any reaction cannot be predicted by merely looking at the balanced chemical equation, i.e., theoretically but must be determined experimentally.
`=>` Conversely, rates generally increase when reactant concentrations increase. So, rate of a reaction depends upon the concentration of reactants.
`=>` Consider a general reaction `aA + bB → c C + d D`
where `a`, `b`, `c` and `d` are the stoichiometric coefficients of reactants and products. The rate expression for this reaction is
Rate `prop [A]^x [B]^y` ..................(1).
where exponents `x` and `y` may or may not be equal to the stoichiometric coefficients (`a` and `b`) of the reactants. Above equation
can also be written as
Rate = `k [A]^x [B]^y` ...............(2).
` - (d [R])/(dt) = k [A]^x [B]^y` ...............(3).
● This form of equation (3) is known as differential rate equation, where `k` is a proportionality constant called rate constant.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
Rate constant may be defined as the rate of the reaction when the molar concentration of each reactant is taken as unity. That is why the rate constant is also called specific reaction rate.
`color{green} ✍️ color{green} mathbf("Characteristics of rate constant")`:
•Greater is the value of the rate constant, faster is the reaction.
•Each reaction has a definite value of the rate constant at a particular temperature.
•The value of the rate constant for the same reaction changes with temperature.
•The value of the rate constant of a reaction does not depend upon the concentrations of the reactants.
•The units of the rate constant depends upon the order of the reaction.
`text(Rate Law of Rate Expression) :` The equation which relates the rate of a reaction to concentration of reactants is called rate law or rate expression.
● Thus, rate law is the expression in which reaction rate is given in terms of molar concentration of reactants with each term raised to some power, which may or may not be same as the stoichiometric coefficient of the reacting species in a balanced chemical equation.
● For example :
`2NO(g) + O_2 (g) → 2NO_2 (g)`
We can measure the rate of this reaction as a function of initial concentrations either by keeping the concentration of one of the reactants constant and changing the concentration of the other reactant or by changing the concentration of both the reactants.
The following results are obtained:
`->` It is obvious, after looking at the results, that when the concentration of `NO` is doubled and that of `O_2` is kept constant then the initial rate increases by a factor of four from `0.096` to `0.384 mol L^(–1)s^(–1)`.
`->` This indicates that the rate depends upon the square of the concentration of `NO`.
`->` When concentration of `NO` is kept constant and concentration of `O_2` is doubled the rate also gets doubled indicating that rate depends on concentration of `O_2` to the first power.
`->` Hence, the rate equation for this reaction will be
Rate `= k[NO]_2[O_2]`
`->` The differential form of this rate expression is given as :
`- (d [R])/(dt) = k [NO]^2 [O_2]`
`->` Here, we observe that for this reaction in the rate equation derived from the experimental data, the exponents of the concentration terms are the same as their stoichiometric coefficients in the balanced chemical equation.
● Some other examples are given below :
`->` In these reactions, the exponents of the concentration terms are not the same as their stoichiometric coefficients.
`=>` Thus, we can say that Rate law for any reaction cannot be predicted by merely looking at the balanced chemical equation, i.e., theoretically but must be determined experimentally.