Temperature Dependence of the Rate of a Reaction :
All the collisions are not effective. The colliding molecules must have energy more than a particular value.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
The minimum energy which the colliding molecules must have in order that the collision between them may be effective is called threshold energy.
At room temperature, most of the reactant molecules have energy less than the threshold value. However, if energy is supplied in the form of heat, light,etc., the reactant molecules absorb this energy and their energy becomes equal to or greater than threshold value. Hence, they start reacting and change into products.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
The minimum extra amount of energy absorbed by the reactant molecules so their energy becomes equal to threshold value is called activation energy.Or it can be said that:
Activation energy=Threshold energy-Average kinetic energy of the reactants
`color { maroon} ® color{maroon} ul (" REMEMBER")`
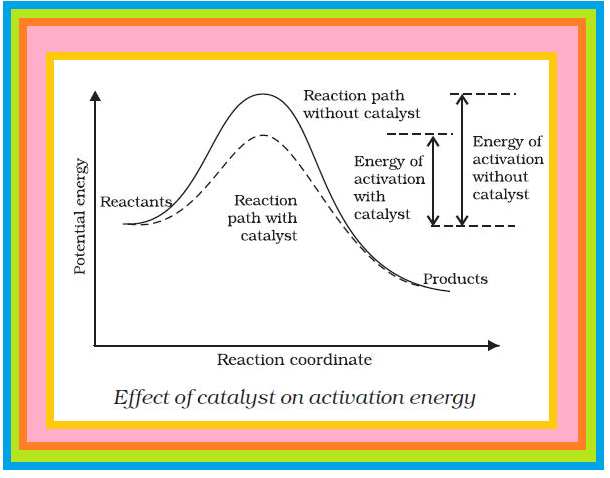
Less is the activation energy,faster is the reaction or greater is the activation energy,slower is the reaction.(FIG. B)
`=>` Most of the chemical reactions are accelerated by increase in temperature.
`color{red}("Example") :` (i) In decomposition of `color{red}(N_2O_5)`, the time taken for half of the original amount of material to decompose is `12` min at `50^oC`, `5` h at `25^oC` and `10` days at `0^oC`.
(ii) In a mixture of potassium permanganate `color{red}((KMnO_4))` and oxalic acid `color{red}((H_2C_2O_4))`, potassium permanganate gets decolourised faster at a higher temperature than that at a lower temperature.
`=>` It has been found that for a chemical reaction with rise in temperature by `10^0`, the rate constant is nearly doubled.
`color{green}("Arrhenius Equation") :` The temperature dependence of the rate of a chemical reaction can be accurately explained by Arrhenius equation (1).
`=>` It was first proposed by Dutch chemist, J. H. van’t Hoff but Swedish chemist, Arrhenius provided its physical justification and interpretation.
`color{red}(k = A e^(-E_a//RT))` ..........(1)
`●` `color{red}(A)` is the Arrhenius factor or the frequency factor. It is also called pre-exponential factor. It is a constant specific to a particular reaction.
`●` `color{red}(R)` is gas constant
`●` `color{red}(E_a)` is activation energy measured in joules/mole `color{red}(J mol^( –1))`.
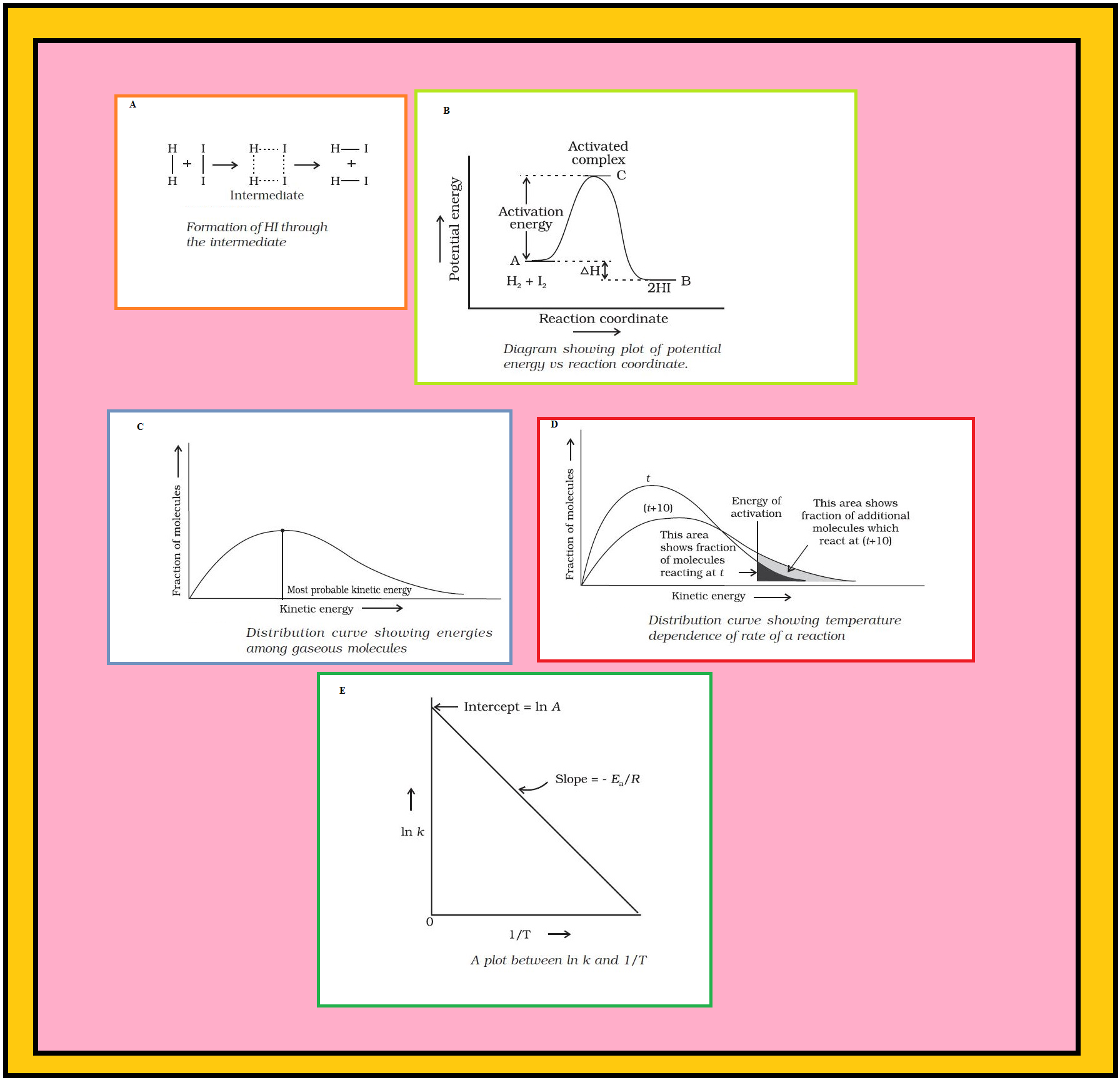
`color{red}("Example") :` It can be understood by following simple reaction `color{red}(H_2(g) +I_2 (g) → 2HI (g))`
`=>` According to Arrhenius, this reaction can take place only when a molecule of hydrogen and a molecule of iodine collide to form an unstable intermediate (Fig.A).
`=>` It exists for a very short time and then breaks up to form two molecules of hydrogen iodide.
`=>` The energy required to form this intermediate, called activated complex (`color{red}(C)`), is known as activation energy (`color{red}(E_a)`).
`=>` Fig. B is obtained by plotting potential energy vs reaction coordinate.
`=>` Reaction coordinate represents the profile of energy change when reactants change into products.
`=>` Some energy is released when the complex decomposes to form products. So, the final heat of the reaction depends upon the nature of reactants and products.
`color{green} ✍️ color{green} mathbf("KEY CONCEPT")`
For the reactants to change into products, they have to cross an energy barrier. Reaction coordinates represent the reaction profiles,i.e., progress of reaction from from reactants to products accompanied by changes in potential energy.
When the reactant molecules absorb energy, their bonds are are loosened and new loose bonds are formed between them. The intermediate thus formed is called an activated complex or transition state complex. It is unstable and immediately dissociates to form the stable products.
`=>` All the molecules in the reacting species do not have the same kinetic energy.
`=>` Since it is difficult to predict the behaviour of any one molecule with precision, Ludwig Boltzmann and James Clark Maxwell used statistics to predict the behaviour of large number of molecules.
`=>` According to them, the distribution of kinetic energy may be described by plotting the fraction of molecules `(color{red}(N_E/N_T))` with a given kinetic energy `(color{red}(E))` vs kinetic energy (Fig.). Here, `color{red}(N_E)` is the number of molecules with energy `color{red}(E)` and `color{red}(N_T)` is total number of molecules.
`=>` The peak of the curve corresponds to the most probable kinetic energy, i.e., kinetic energy of maximum fraction of molecules.
`=>` There are decreasing number of molecules with energies higher or lower than this value.
`=>` When the temperature is raised, the maximum of the curve moves to the higher energy value (Fig.) and the curve broadens out, i.e., spreads to the right such that there is a greater proportion of molecules with much higher energies.
`=>` The area under the curve must be constant since total probability must be one at all times.
`=>` We can mark the position of `color{red}(E_a)` on Maxwell Boltzmann distribution curve (Fig.D).
`=>` Increasing the temperature of the substance increases the fraction of molecules, which collide with energies greater than `color{red}(E_a)`.
`=>` It is clear from the diagram that in the curve at `color{red}((t + 10))`, the area showing the fraction of molecules having energy equal to or greater than activation energy gets doubled leading to doubling the rate of a reaction.
`=>` In the Arrhenius equation (1) the factor `color{red}(e^(-E_a //RT))` corresponds to the fraction of molecules that have kinetic energy greater than `color{red}(E_a)`. Taking natural logarithm of both sides of equation (1)
`color{red}(ln k = - (E_a)/(RT) + ln A)` .................(2)
`=>` The plot of `color{red}(ln k vs 1/T)` gives a straight line according to the equation (2) as shown in Fig.E.
`=>` Thus, from Arrhenius equation (4.18) it is concluded that increasing the temperature or decreasing the activation energy will result in an increase in the rate of the reaction and an exponential increase in the rate constant.
`=>` In Fig., `color{red}(text(slope) = - (E_a)/R)` and intercept `color{red}(= ln A)`. So we can calculate `color{red}(E_a)` and `color{red}(A)` using these values.
`=>` At temperature `color{red}(T_1)`, equation (2) is
`color{red}(ln k_1 = - E_a/(RT_1) + ln A)` ................(3)
At temperature `color{red}(T_2)`, equation (2) is
`color{red}(ln k_2 = - E_a/(RT_2) + ln A) ` ...............(4)
(since `color{red}(A)` is constant for a given reaction)
`color{red}(k_1)` and `color{red}(k_2)` are the values of rate constants at temperatures `color{red}(T_1)` and `color{red}(T_2)` respectively. Subtracting equation (3) from (4), we obtain
`color{red}(ln k_2 - ln k_1 = E_a/(RT_1) - E_a/(RT_2))`
`color{red}(ln \ \ k_2/k_1 = E_a/R [ 1/T_1 - 1/T_2])` ...............(5)
`color{red}(log \ \ k_2/k_1 = E_a/(2.303 R) [ (T_2-T_1)/(T_1 T_2)])`
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
The minimum energy which the colliding molecules must have in order that the collision between them may be effective is called threshold energy.
At room temperature, most of the reactant molecules have energy less than the threshold value. However, if energy is supplied in the form of heat, light,etc., the reactant molecules absorb this energy and their energy becomes equal to or greater than threshold value. Hence, they start reacting and change into products.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
The minimum extra amount of energy absorbed by the reactant molecules so their energy becomes equal to threshold value is called activation energy.Or it can be said that:
Activation energy=Threshold energy-Average kinetic energy of the reactants
`color { maroon} ® color{maroon} ul (" REMEMBER")`
Less is the activation energy,faster is the reaction or greater is the activation energy,slower is the reaction.(FIG. B)
`=>` Most of the chemical reactions are accelerated by increase in temperature.
`color{red}("Example") :` (i) In decomposition of `color{red}(N_2O_5)`, the time taken for half of the original amount of material to decompose is `12` min at `50^oC`, `5` h at `25^oC` and `10` days at `0^oC`.
(ii) In a mixture of potassium permanganate `color{red}((KMnO_4))` and oxalic acid `color{red}((H_2C_2O_4))`, potassium permanganate gets decolourised faster at a higher temperature than that at a lower temperature.
`=>` It has been found that for a chemical reaction with rise in temperature by `10^0`, the rate constant is nearly doubled.
`color{green}("Arrhenius Equation") :` The temperature dependence of the rate of a chemical reaction can be accurately explained by Arrhenius equation (1).
`=>` It was first proposed by Dutch chemist, J. H. van’t Hoff but Swedish chemist, Arrhenius provided its physical justification and interpretation.
`color{red}(k = A e^(-E_a//RT))` ..........(1)
`●` `color{red}(A)` is the Arrhenius factor or the frequency factor. It is also called pre-exponential factor. It is a constant specific to a particular reaction.
`●` `color{red}(R)` is gas constant
`●` `color{red}(E_a)` is activation energy measured in joules/mole `color{red}(J mol^( –1))`.
`color{red}("Example") :` It can be understood by following simple reaction `color{red}(H_2(g) +I_2 (g) → 2HI (g))`
`=>` According to Arrhenius, this reaction can take place only when a molecule of hydrogen and a molecule of iodine collide to form an unstable intermediate (Fig.A).
`=>` It exists for a very short time and then breaks up to form two molecules of hydrogen iodide.
`=>` The energy required to form this intermediate, called activated complex (`color{red}(C)`), is known as activation energy (`color{red}(E_a)`).
`=>` Fig. B is obtained by plotting potential energy vs reaction coordinate.
`=>` Reaction coordinate represents the profile of energy change when reactants change into products.
`=>` Some energy is released when the complex decomposes to form products. So, the final heat of the reaction depends upon the nature of reactants and products.
`color{green} ✍️ color{green} mathbf("KEY CONCEPT")`
For the reactants to change into products, they have to cross an energy barrier. Reaction coordinates represent the reaction profiles,i.e., progress of reaction from from reactants to products accompanied by changes in potential energy.
When the reactant molecules absorb energy, their bonds are are loosened and new loose bonds are formed between them. The intermediate thus formed is called an activated complex or transition state complex. It is unstable and immediately dissociates to form the stable products.
`=>` All the molecules in the reacting species do not have the same kinetic energy.
`=>` Since it is difficult to predict the behaviour of any one molecule with precision, Ludwig Boltzmann and James Clark Maxwell used statistics to predict the behaviour of large number of molecules.
`=>` According to them, the distribution of kinetic energy may be described by plotting the fraction of molecules `(color{red}(N_E/N_T))` with a given kinetic energy `(color{red}(E))` vs kinetic energy (Fig.). Here, `color{red}(N_E)` is the number of molecules with energy `color{red}(E)` and `color{red}(N_T)` is total number of molecules.
`=>` The peak of the curve corresponds to the most probable kinetic energy, i.e., kinetic energy of maximum fraction of molecules.
`=>` There are decreasing number of molecules with energies higher or lower than this value.
`=>` When the temperature is raised, the maximum of the curve moves to the higher energy value (Fig.) and the curve broadens out, i.e., spreads to the right such that there is a greater proportion of molecules with much higher energies.
`=>` The area under the curve must be constant since total probability must be one at all times.
`=>` We can mark the position of `color{red}(E_a)` on Maxwell Boltzmann distribution curve (Fig.D).
`=>` Increasing the temperature of the substance increases the fraction of molecules, which collide with energies greater than `color{red}(E_a)`.
`=>` It is clear from the diagram that in the curve at `color{red}((t + 10))`, the area showing the fraction of molecules having energy equal to or greater than activation energy gets doubled leading to doubling the rate of a reaction.
`=>` In the Arrhenius equation (1) the factor `color{red}(e^(-E_a //RT))` corresponds to the fraction of molecules that have kinetic energy greater than `color{red}(E_a)`. Taking natural logarithm of both sides of equation (1)
`color{red}(ln k = - (E_a)/(RT) + ln A)` .................(2)
`=>` The plot of `color{red}(ln k vs 1/T)` gives a straight line according to the equation (2) as shown in Fig.E.
`=>` Thus, from Arrhenius equation (4.18) it is concluded that increasing the temperature or decreasing the activation energy will result in an increase in the rate of the reaction and an exponential increase in the rate constant.
`=>` In Fig., `color{red}(text(slope) = - (E_a)/R)` and intercept `color{red}(= ln A)`. So we can calculate `color{red}(E_a)` and `color{red}(A)` using these values.
`=>` At temperature `color{red}(T_1)`, equation (2) is
`color{red}(ln k_1 = - E_a/(RT_1) + ln A)` ................(3)
At temperature `color{red}(T_2)`, equation (2) is
`color{red}(ln k_2 = - E_a/(RT_2) + ln A) ` ...............(4)
(since `color{red}(A)` is constant for a given reaction)
`color{red}(k_1)` and `color{red}(k_2)` are the values of rate constants at temperatures `color{red}(T_1)` and `color{red}(T_2)` respectively. Subtracting equation (3) from (4), we obtain
`color{red}(ln k_2 - ln k_1 = E_a/(RT_1) - E_a/(RT_2))`
`color{red}(ln \ \ k_2/k_1 = E_a/R [ 1/T_1 - 1/T_2])` ...............(5)
`color{red}(log \ \ k_2/k_1 = E_a/(2.303 R) [ (T_2-T_1)/(T_1 T_2)])`