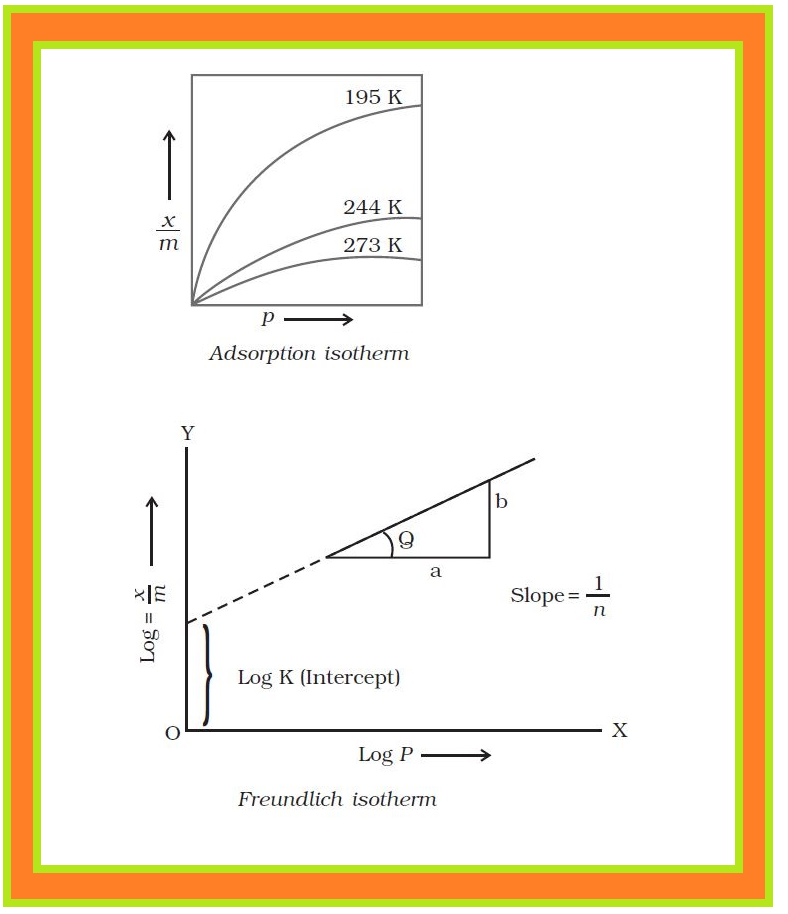
`text(Definition) :` The variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve termed as adsorption isotherm.
`text(Freundlich Adsorption Isotherm :)` Freundlich, in 1909, gave an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature. The relationship can be expressed by the following equation :
`x/m = kp^(1/n) ( n > 1)` ...........(1).
● where `x` is the mass of the gas adsorbed on mass `m` of the adsorbent at pressure `P`,
● `k` and `n` are constants which depend on the nature of the adsorbent and the gas at a particular temperature.
`=>` The relationship is generally represented in the form of a curve where mass of the gas adsorbed per gram of the adsorbent is plotted against pressure.
`=>` These curves indicate that at a fixed pressure, there is a decrease in physical adsorption with increase in temperature. These curves always seem to approach saturation at high pressure.
Taking logarithm of eq. (1)
`log \ \ x/m = log k +1/n log P` .................(2)
`=>` The validity of Freundlich isotherm can be verified by plotting `log \ \ x/m` on y-axis (ordinate) and `log P` on `x`-axis (abscissa).
● If it comes to be a straight line, the Freundlich isotherm is valid, otherwise not.
● The slope of the straight line gives the value of `1/n`. The intercept on the y-axis gives the value of `log k`.
`=>` Freundlich isotherm explains the behaviour of adsorption in an approximate manner.
`=>` The factor `1/n` can have values between `0` and `1` (probable range `0.1` to `0.5`). Thus, equation (2) holds good over a limited range of pressure.
● when `1/n = 0 , x/m = ` constant, the adsorption is independent of pressure.
● when `1/n = 1 , x/m = k P , ` i.e `x/m prop P` the adsorption varies directly with pressure.
Both the conditions are supported by experimental results. The experimental isotherms always seem to approach saturation at high pressure. This cannot be explained by Freundlich isotherm. Thus, it fails at high pressure.
`text(Definition) :` The variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve termed as adsorption isotherm.
`text(Freundlich Adsorption Isotherm :)` Freundlich, in 1909, gave an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature. The relationship can be expressed by the following equation :
`x/m = kp^(1/n) ( n > 1)` ...........(1).
● where `x` is the mass of the gas adsorbed on mass `m` of the adsorbent at pressure `P`,
● `k` and `n` are constants which depend on the nature of the adsorbent and the gas at a particular temperature.
`=>` The relationship is generally represented in the form of a curve where mass of the gas adsorbed per gram of the adsorbent is plotted against pressure.
`=>` These curves indicate that at a fixed pressure, there is a decrease in physical adsorption with increase in temperature. These curves always seem to approach saturation at high pressure.
Taking logarithm of eq. (1)
`log \ \ x/m = log k +1/n log P` .................(2)
`=>` The validity of Freundlich isotherm can be verified by plotting `log \ \ x/m` on y-axis (ordinate) and `log P` on `x`-axis (abscissa).
● If it comes to be a straight line, the Freundlich isotherm is valid, otherwise not.
● The slope of the straight line gives the value of `1/n`. The intercept on the y-axis gives the value of `log k`.
`=>` Freundlich isotherm explains the behaviour of adsorption in an approximate manner.
`=>` The factor `1/n` can have values between `0` and `1` (probable range `0.1` to `0.5`). Thus, equation (2) holds good over a limited range of pressure.
● when `1/n = 0 , x/m = ` constant, the adsorption is independent of pressure.
● when `1/n = 1 , x/m = k P , ` i.e `x/m prop P` the adsorption varies directly with pressure.
Both the conditions are supported by experimental results. The experimental isotherms always seem to approach saturation at high pressure. This cannot be explained by Freundlich isotherm. Thus, it fails at high pressure.