Adsorption Theory of Heterogeneous Catalysis :
`=>` This theory explains the mechanism of heterogeneous catalysis.
`=>` The old theory, known as adsorption theory of catalysis, was that the reactants in gaseous state or in solutions, are adsorbed on the surface of the solid catalyst.
● The increase in concentration of the reactants on the surface increases the rate of reaction.
● Adsorption being an exothermic process, the heat of adsorption is utilised in enhancing the rate of the reaction.
● The catalytic action can be explained in terms of the intermediate compound formation.
`=>` The modern adsorption theory is the combination of intermediate compound formation theory and the old adsorption theory. The catalytic activity is localised on the surface of the catalyst.
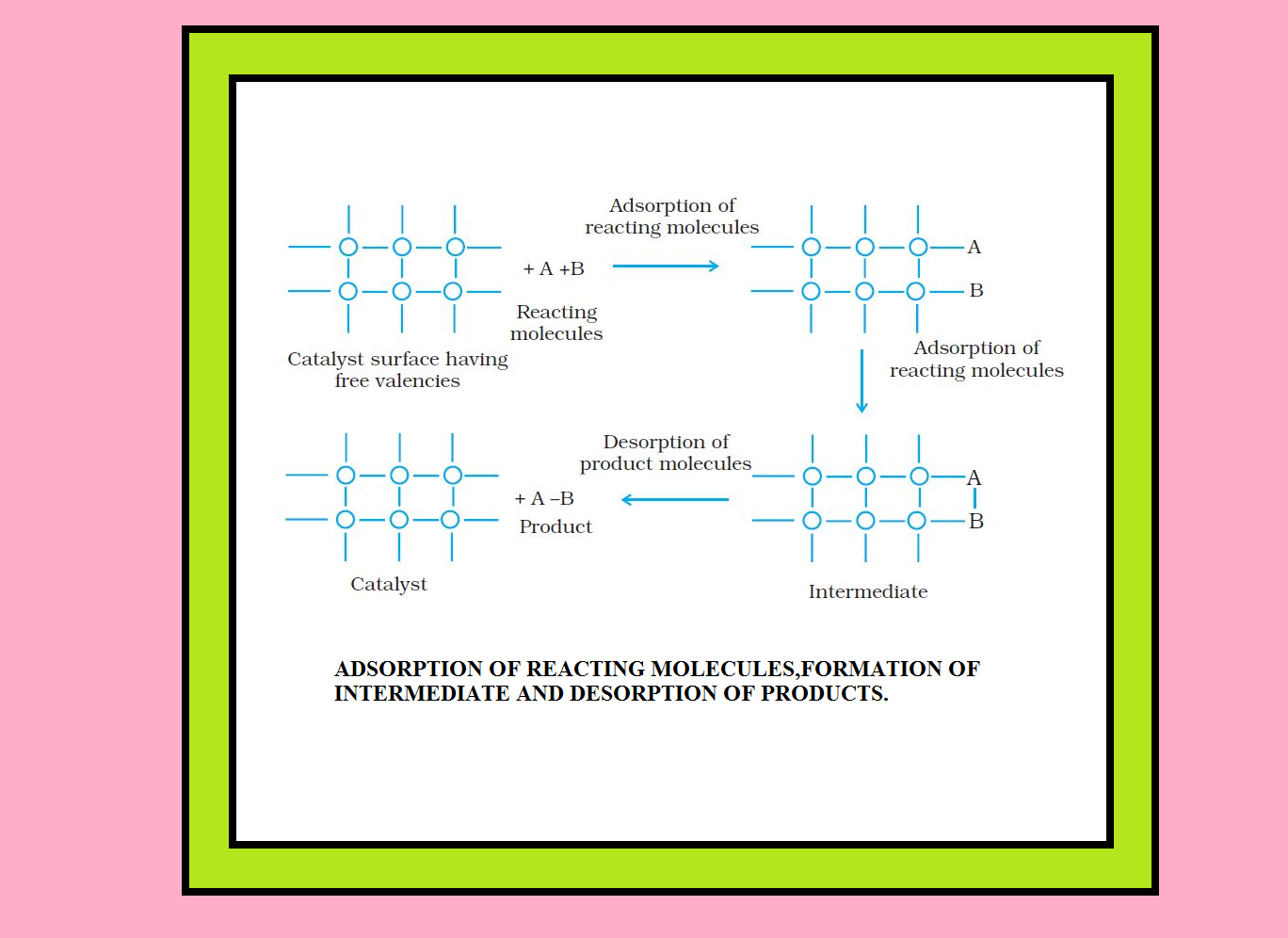
`=>` The mechanism involves five steps :
(i) Diffusion of reactants to the surface of the catalyst.
(ii) Adsorption of reactant molecules on the surface of the catalyst.
(iii) Occurrence of chemical reaction on the catalyst’s surface through formation of an intermediate.
(iv) Desorption of reaction products from the catalyst surface, and thereby, making the surface available again for more reaction to occur.
(v) Diffusion of reaction products away from the catalyst’s surface.
● The surface of the catalyst unlike the inner part of the bulk, has free valencies which provide the seat for chemical forces of attraction.
● When a gas comes in contact with such a surface, its molecules are held up there due to loose chemical combination.
● If different molecules are adsorbed side by side, they may react with each other resulting in the formation of new molecules.
● Thus, formed molecules may evaporate leaving the surface for the fresh reactant molecules.
`=>` This theory explains why the catalyst remains unchanged in mass and chemical composition at the end of the reaction and is effective even in small quantities.
`=>` It however, does not explain the action of catalytic promoters and catalytic poisons.
`=>` The old theory, known as adsorption theory of catalysis, was that the reactants in gaseous state or in solutions, are adsorbed on the surface of the solid catalyst.
● The increase in concentration of the reactants on the surface increases the rate of reaction.
● Adsorption being an exothermic process, the heat of adsorption is utilised in enhancing the rate of the reaction.
● The catalytic action can be explained in terms of the intermediate compound formation.
`=>` The modern adsorption theory is the combination of intermediate compound formation theory and the old adsorption theory. The catalytic activity is localised on the surface of the catalyst.
`=>` The mechanism involves five steps :
(i) Diffusion of reactants to the surface of the catalyst.
(ii) Adsorption of reactant molecules on the surface of the catalyst.
(iii) Occurrence of chemical reaction on the catalyst’s surface through formation of an intermediate.
(iv) Desorption of reaction products from the catalyst surface, and thereby, making the surface available again for more reaction to occur.
(v) Diffusion of reaction products away from the catalyst’s surface.
● The surface of the catalyst unlike the inner part of the bulk, has free valencies which provide the seat for chemical forces of attraction.
● When a gas comes in contact with such a surface, its molecules are held up there due to loose chemical combination.
● If different molecules are adsorbed side by side, they may react with each other resulting in the formation of new molecules.
● Thus, formed molecules may evaporate leaving the surface for the fresh reactant molecules.
`=>` This theory explains why the catalyst remains unchanged in mass and chemical composition at the end of the reaction and is effective even in small quantities.
`=>` It however, does not explain the action of catalytic promoters and catalytic poisons.