Colloids :
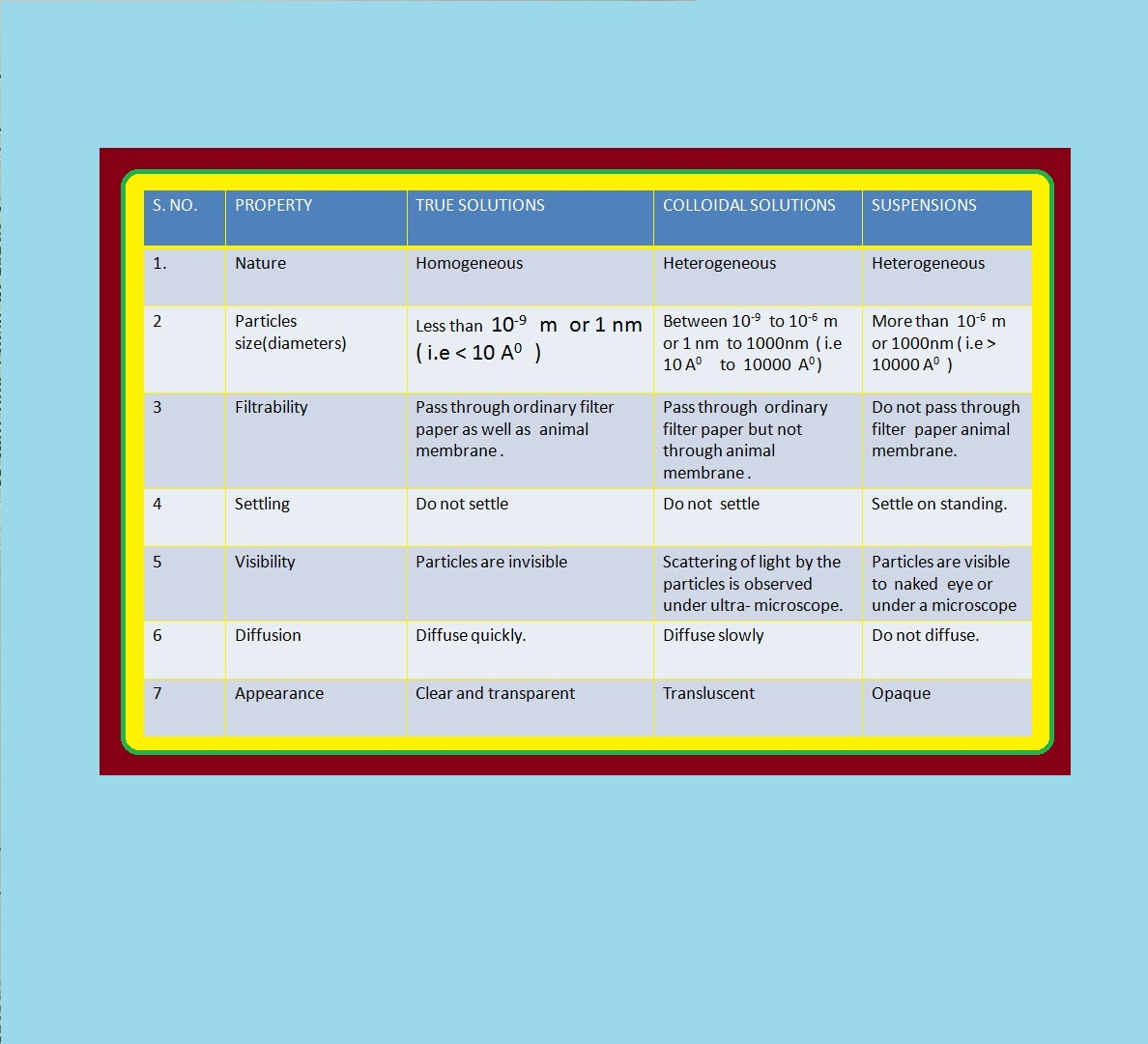
`=>` We know that solutions are homogeneous systems.
`=>` We know that sand in water when stirred gives a suspension, which slowly settles down with time.
`=>` Between the two extremes of suspensions and solutions we come across a large group of systems called colloidal dispersions or simply colloids.
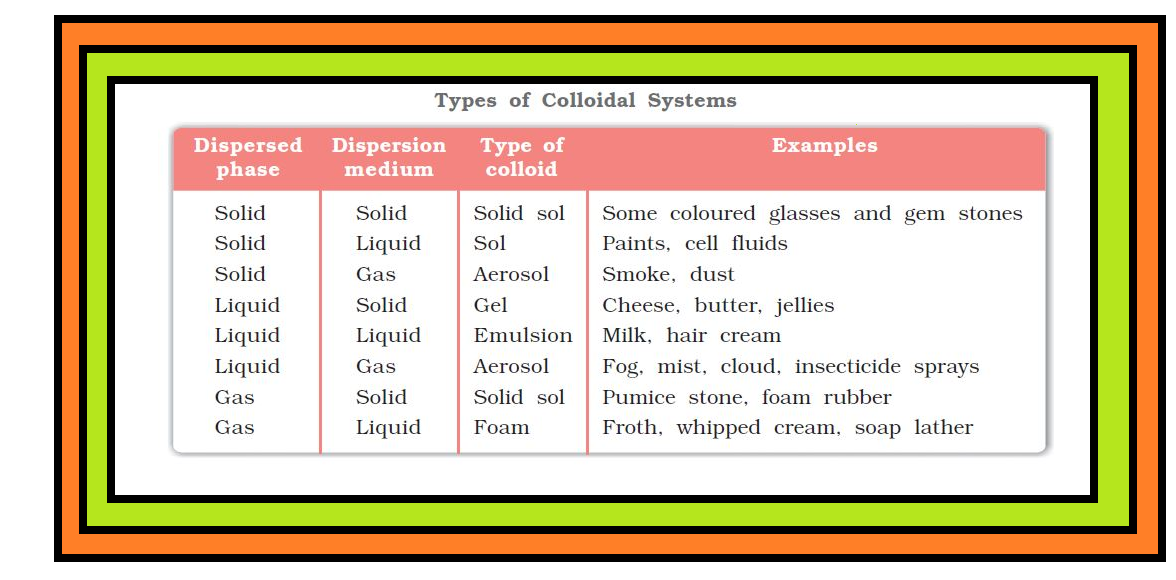
`text(Definition :)` A colloid is a heterogeneous system in which one substance is dispersed (dispersed phase) as very fine particles in another substance called dispersion medium.
`=>` The essential difference between a solution and a colloid is that of particle size.
`=>` In a solution, the constituent particles are ions or small molecules.
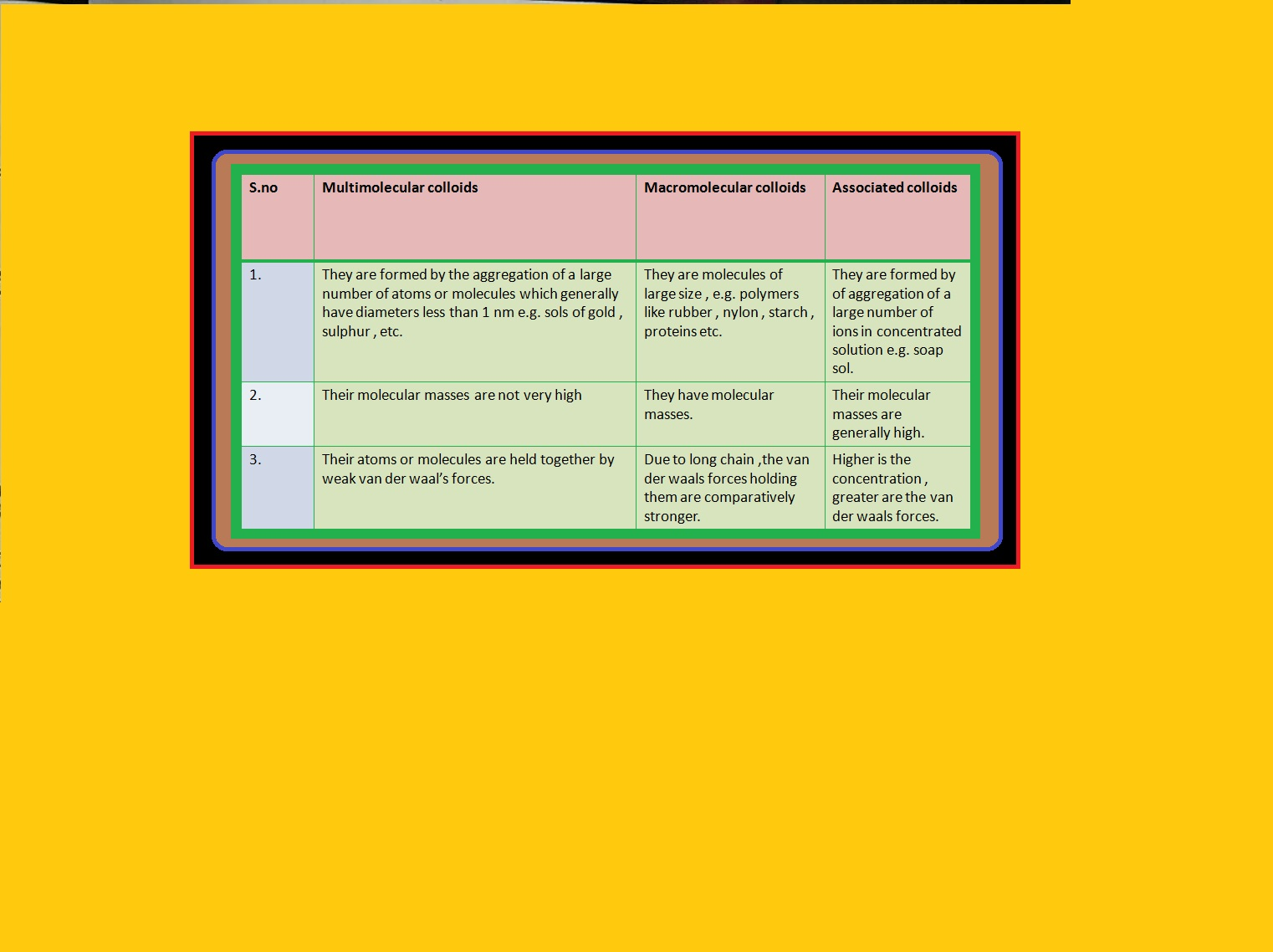
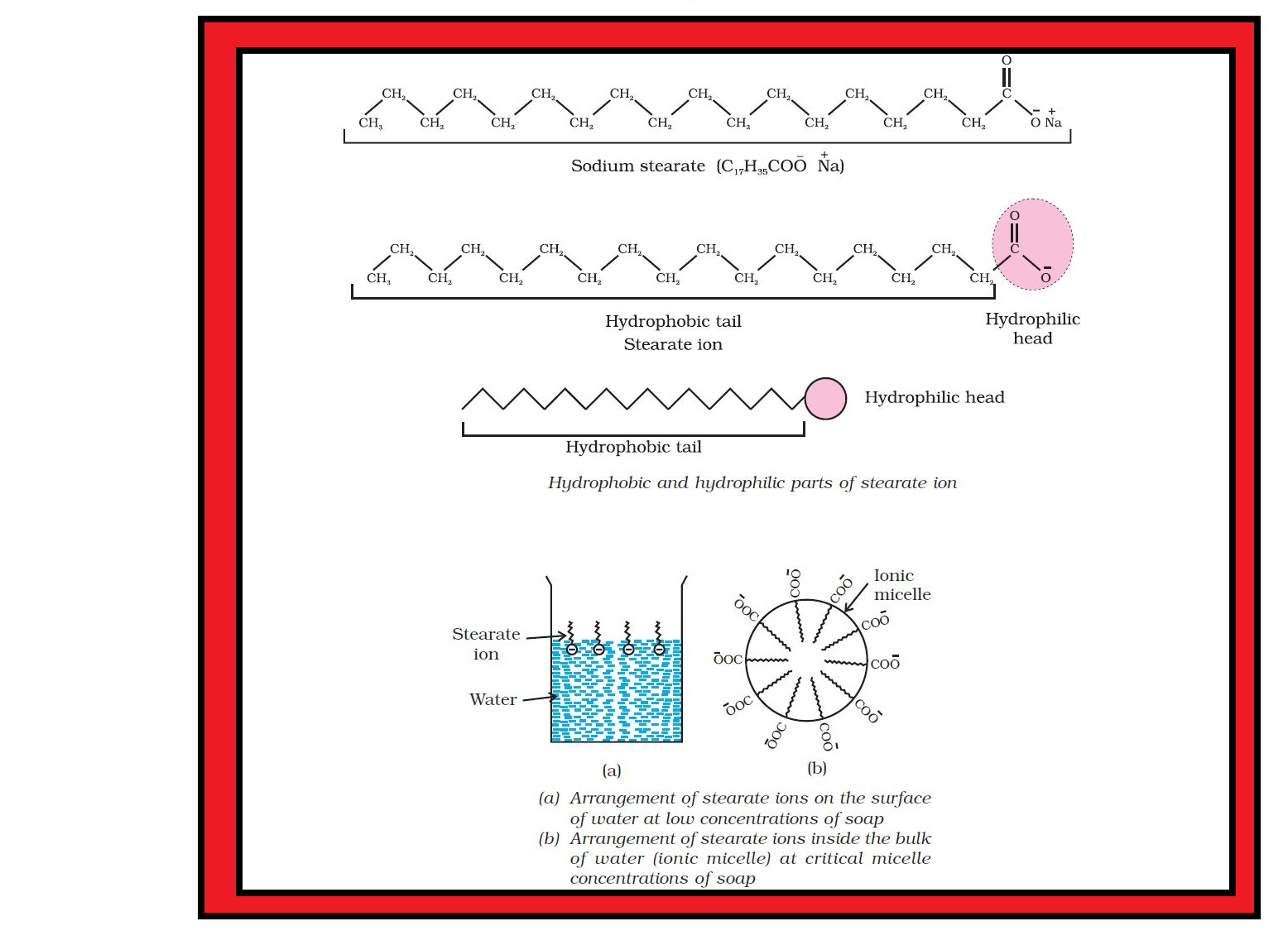
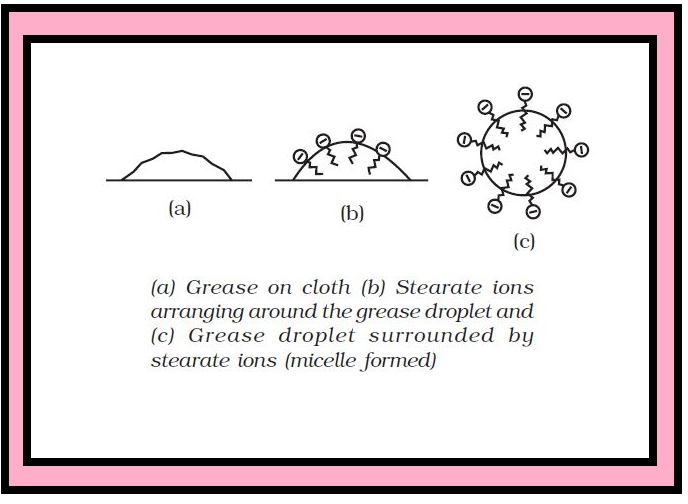
`=>` In a colloid, the dispersed phase may consist of particles of a single macromolecule (such as protein or synthetic polymer) or an aggregate of many atoms, ions or molecules.
`=>` Colloidal particles are larger than simple molecules but small enough to remain suspended.
`=>` Their range of diameters is between `1` and `1000` nm (`10^(–9)` to `10^(–6) m`).
`=>` Colloidal particles have an enormous surface area per unit mass as a result of their small size.
`=>` Consider a cube with 1 cm side. It has a total surface area of `6 cm^2`. If it were divided equally into 1012 cubes, the cubes would be the size of large colloidal particles and have a total surface area of `60,000 cm^2` or `6 m^2`.
`=>` We know that sand in water when stirred gives a suspension, which slowly settles down with time.
`=>` Between the two extremes of suspensions and solutions we come across a large group of systems called colloidal dispersions or simply colloids.
`text(Definition :)` A colloid is a heterogeneous system in which one substance is dispersed (dispersed phase) as very fine particles in another substance called dispersion medium.
`=>` The essential difference between a solution and a colloid is that of particle size.
`=>` In a solution, the constituent particles are ions or small molecules.
`=>` In a colloid, the dispersed phase may consist of particles of a single macromolecule (such as protein or synthetic polymer) or an aggregate of many atoms, ions or molecules.
`=>` Colloidal particles are larger than simple molecules but small enough to remain suspended.
`=>` Their range of diameters is between `1` and `1000` nm (`10^(–9)` to `10^(–6) m`).
`=>` Colloidal particles have an enormous surface area per unit mass as a result of their small size.
`=>` Consider a cube with 1 cm side. It has a total surface area of `6 cm^2`. If it were divided equally into 1012 cubes, the cubes would be the size of large colloidal particles and have a total surface area of `60,000 cm^2` or `6 m^2`.