Preparation of Colloids :
A few important methods for the preparation of colloids are as follows:
(a) `text(Chemical Methods :)` Colloidal solutions can be prepared by chemical reactions leading to formation of molecules by double decomposition, oxidation, reduction or hydrolysis. These molecules then aggregate leading to formation of sols.
`AS_2 O_3 +3H_2S oversettext(Double decompostion)→ As_2S_3 (Sol) +3H_2O`
`SO_2+2H_2S oversettext(Oxidation)→ 3S (Sol)+2H_2O`
`2AuCl_3+3HCHO+3H_2O oversettext(Reduction)→ 2Au (sol)+3HCOOH +6HCl`
`FeCl_2+3H_2O oversettext(Hydrolysis)→ Fe(OH)_3 (sol) +3HCl`
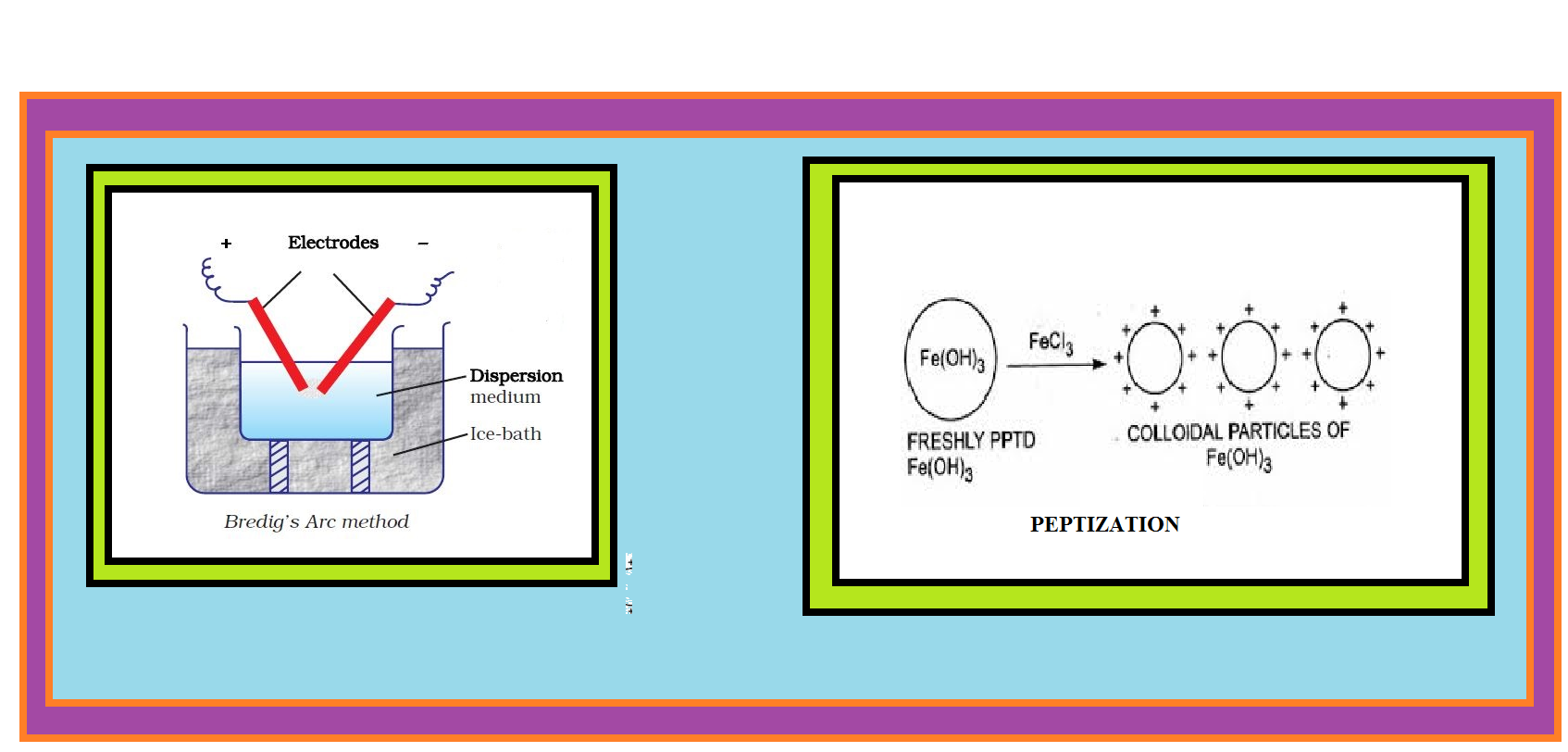
(b) `text(Electrical disintegration or Bredig’s Arc Method :)` This process involves dispersion as well as condensation.
● Colloidal sols of metals such as gold, silver, platinum, etc., can be prepared by this method.
● In this method, electric arc is struck between electrodes of the metal immersed in the dispersion medium.
● The intense heat produced vapourises the metal, which then condenses to form particles of colloidal size.
(c) `text(Peptization :)` Peptization may be defined as the process of converting a precipitate into colloidal sol by shaking it with dispersion medium in the presence of a small amount of electrolyte.
● The electrolyte used for this purpose is called peptizing agent.
● This method is applied, generally, to convert a freshly prepared precipitate into a colloidal sol.
● During peptization, the precipitate adsorbs one of the ions of the electrolyte on its surface.
● This causes the development of positive or negative charge on precipitates, which ultimately break up into smaller particles of the size of a colloid.
(a) `text(Chemical Methods :)` Colloidal solutions can be prepared by chemical reactions leading to formation of molecules by double decomposition, oxidation, reduction or hydrolysis. These molecules then aggregate leading to formation of sols.
`AS_2 O_3 +3H_2S oversettext(Double decompostion)→ As_2S_3 (Sol) +3H_2O`
`SO_2+2H_2S oversettext(Oxidation)→ 3S (Sol)+2H_2O`
`2AuCl_3+3HCHO+3H_2O oversettext(Reduction)→ 2Au (sol)+3HCOOH +6HCl`
`FeCl_2+3H_2O oversettext(Hydrolysis)→ Fe(OH)_3 (sol) +3HCl`
(b) `text(Electrical disintegration or Bredig’s Arc Method :)` This process involves dispersion as well as condensation.
● Colloidal sols of metals such as gold, silver, platinum, etc., can be prepared by this method.
● In this method, electric arc is struck between electrodes of the metal immersed in the dispersion medium.
● The intense heat produced vapourises the metal, which then condenses to form particles of colloidal size.
(c) `text(Peptization :)` Peptization may be defined as the process of converting a precipitate into colloidal sol by shaking it with dispersion medium in the presence of a small amount of electrolyte.
● The electrolyte used for this purpose is called peptizing agent.
● This method is applied, generally, to convert a freshly prepared precipitate into a colloidal sol.
● During peptization, the precipitate adsorbs one of the ions of the electrolyte on its surface.
● This causes the development of positive or negative charge on precipitates, which ultimately break up into smaller particles of the size of a colloid.