Properties of Colloidal Solutions :
Various properties exhibited by the colloidal solutions are described below :
(i) `text(Colligative Properties :)` Colloidal particles being bigger aggregates, the number of particles in a colloidal solution is comparatively small as compared to a true solution. Hence, the values of colligative properties (osmotic pressure, lowering in vapour pressure, depression in freezing point and elevation in boiling point) are of small order as compared to values shown by true solutions at same concentrations.
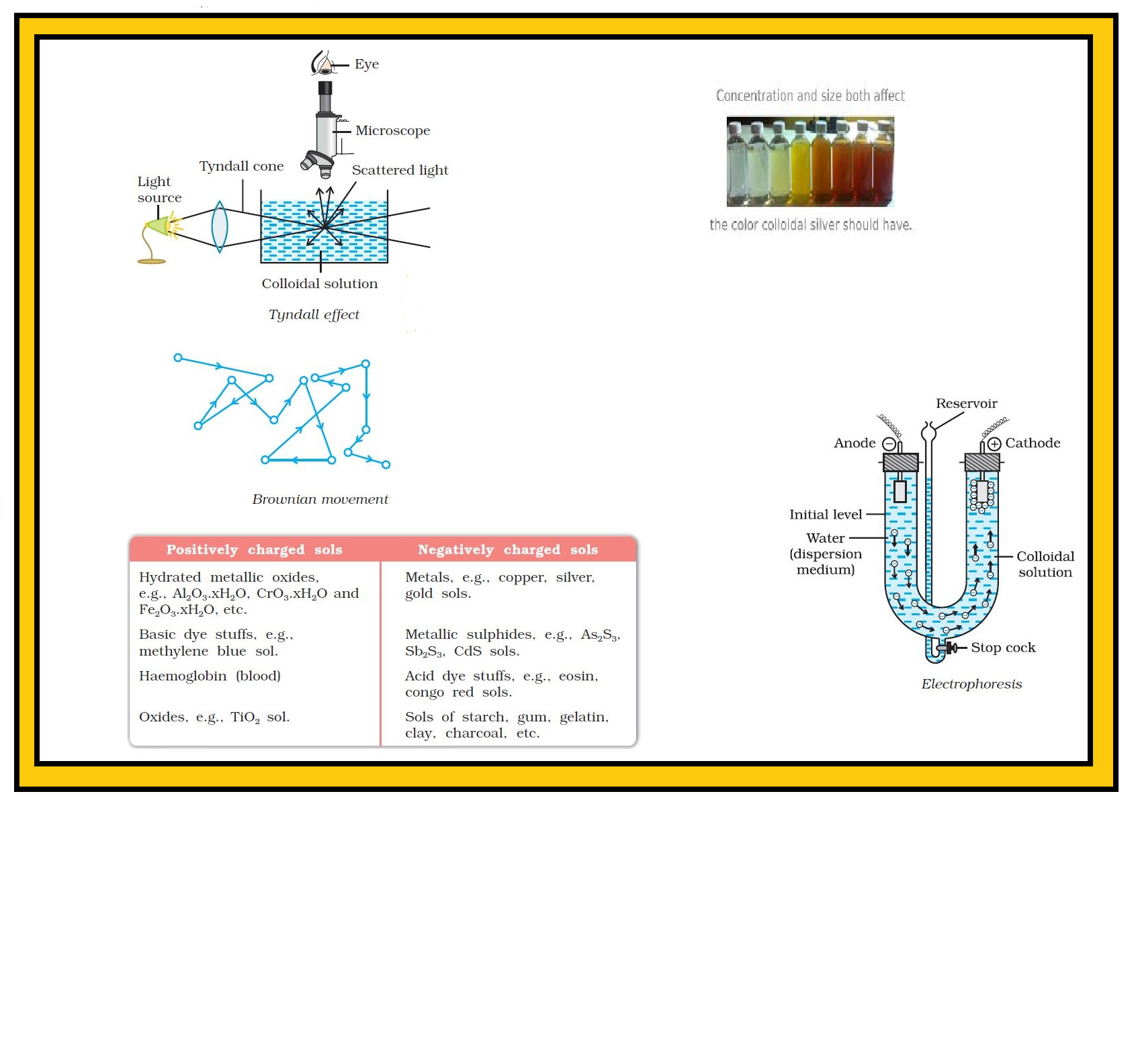
(ii) `text(Tyndall Effect :)`
● If a homogeneous solution placed in dark is observed in the direction of light, it appears clear and, if it is observed from a direction at right angles to the direction of light beam, it appears perfectly dark.
● Colloidal solutions viewed in the same way may also appear reasonably clear or translucent by the transmitted light but they show a mild to strong opalescence, when viewed at right angles to the passage of light, i.e., the path of the beam is illuminated by a bluish light.
● This effect was first observed by Faraday and later studied in detail by Tyndall and is termed as Tyndall effect.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
Tyndall effect may be defined as the scattering of light by colloidal particles present in a colloidal solution.
● The bright cone of the light is called Tyndall cone.
● The Tyndall effect is due to the fact that colloidal particles scatter light in all directions in space.
● This scattering of light illuminates the path of beam in the colloidal dispersion.
● Tyndall effect can be observed during the projection of picture in the cinema hall due to scattering of light by dust and smoke particles present there.
● Tyndall effect is observed only when the following two conditions are satisfied.
(a) The diameter of the dispersed particles is not much smaller than the wavelength of the light used; and
(b) The refractive indices of the dispersed phase and the dispersion medium differ greatly in magnitude.
● Tyndall effect is used to distinguish between a colloidal and true solution.
● Zsigmondy, in 1903, used Tyndall effect to set up an apparatus known as ultramicroscope.
● An intense beam of light is focused on the colloidal solution contained in a glass vessel.
● The focus of the light is then observed with a microscope at right angles to the beam.
● Individual colloidal particles appear as bright stars against a dark background.
● Ultramicroscope does not render the actual colloidal particles visible but only observe the light scattered by them.
● Thus, ultramicroscope does not provide any information about the size and shape of colloidal particles.
(iii) `text(Colour :)`
● The colour of colloidal solution depends on the wavelength of light scattered by the dispersed particles.
● The wavelength of light further depends on the size and nature of the particles.
● The colour of colloidal solution also changes with the manner in which the observer receives the light.
● Example : A mixture of milk and water appears blue when viewed by the reflected light and red when viewed by the transmitted light. Finest gold sol is red in colour; as the size of particles increases, it appears purple, then blue and finally golden.
(iv) `text(Brownian Movement :)`
● When colloidal solutions are viewed under a powerful ultramicroscope, the colloidal particles appear to be in a state of continuous zig-zag motion all over the field of view.
● This motion was first observed by the British botanist, Robert Brown, and is known as Brownian movement.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
Brownian movement may be described as continuous zig-zag movement of the colloidal particles in a colloidal sol.
● This motion is independent of the nature of the colloid but depends on the size of the particles and viscosity of the solution.
● Smaller the size and lesser the viscosity, faster is the motion.
● The Brownian movement has been explained to be due to the unbalanced bombardment of the particles by the molecules of the dispersion medium.
● The Brownian movement has a stirring effect which does not permit the particles to settle and thus, is responsible for the stability of sols.
(v) `text(Charge on Colloidal Particles :)`
● Colloidal particles always carry an electric charge.
● The nature of this charge is the same on all the particles in a given colloidal solution and may be either positive or negative.
● A list of some common sols with the nature of charge on their particles is given in the Table.
● The charge on the sol particles is due to one or more reasons, viz., due to electron capture by sol particles during electrodispersion of metals, due to preferential adsorption of ions from solution and/or due to formulation of electrical double layer. Preferential adsorption of ions is the most accepted reason. The sol particles acquire positive or negative charge by preferential adsorption of `+ve` or `–ve` ions. When two or more ions are present in the dispersion medium, preferential adsorption of the ion common to the colloidal particle usually takes place. This can be explained by taking the following examples :
(a) When silver nitrate solution is added to potassium iodide solution, the precipitated silver iodide adsorbs iodide ions from the dispersion medium and negatively charged colloidal solution results. However, when `KI` solution is added to `AgNO_3` solution, positively charged sol results due to adsorption of `Ag^+` ions from dispersion medium.
`undersettext(Negatively charged)((AgI)//I^(-)) \ \ \ \ \ \ \ undersettext(Positively charged)((AgI)//(Ag^+))`
(b) If `FeCl_3` is added to excess of hot water, a positively charged sol of hydrated ferric oxide is formed due to adsorption of `Fe^(3+)` ions. However, when ferric chloride is added to `NaOH` a negatively charged sol is obtained with adsorption of `OH^-` ions.
`undersettext(Positively charged) (Fe_2O_3 . x H_2O //Fe^(3+)) \ \ \ \ undersettext(Negatively charged) (Fe_2O_3 . x H_2O //OH^-)`
● Having acquired a positive or a negative charge by selective adsorption on the surface of a colloidal particle as stated above, this layer attracts counter ions from the medium forming a second layer, as shown below.
`AgI//I^- K^+ \ \ \ \ \ \ \ \ \ AgI//Ag^+ I^-`
● The combination of the two layers of opposite charges around the colloidal particle is called Helmholtz electrical double layer.
● According to modern views, the first layer of ions is firmly held and is termed fixed layer while the second layer is mobile which is termed diffused layer.
● Since separation of charge is a seat of potential, the charges of opposite signs on the fixed and diffused parts of the double layer results in a difference in potential between these layers.
● This potential difference between the fixed layer and the diffused layer of opposite charges is called the electrokinetic potential or zeta potential.
● The presence of equal and similar charges on colloidal particles is largely responsible in providing stability to the colloidal solution, because the repulsive forces between charged particles having same charge prevent them from coalescing or aggregating when they come closer to one another.
(vi) `text(Electrophoresis :)` The existence of charge on colloidal particles is confirmed by electrophoresis experiment. When electric potential is applied across two platinum electrodes dipping in a colloidal solution, the colloidal particles move towards one or the other electrode.
● The movement of colloidal particles under an applied electric potential is called electrophoresis. Positively charged particles move towards the cathode while negatively charged particles move towards the anode.
● This can be demonstrated by the following experimental setup.
● When electrophoresis, i.e., movement of particles is prevented by some suitable means, it is observed that the dispersion medium begins to move in an electric field. This phenomenon is termed electroosmosis.
(vii) `text(Coagulation or Precipitation :)` The stability of the lyophobic sols is due to the presence of charge on colloidal particles. If, somehow, the charge is removed, the particles will come nearer to each other to form aggregates (or coagulate) and settle down under the force of gravity.
The process of settling of colloidal particles is called coagulation or precipitation of the sol. The coagulation of the lyophobic sols can be carried out in the following ways :
(a) By electrophoresis : The colloidal particles move towards oppositely charged electrodes, get discharged and precipitated.
(b) By mixing two oppositely charged sols : Oppositely charged sols when mixed in almost equal proportions, neutralise their charges and get partially or completely precipitated. Mixing of hydrated ferric oxide (+ve sol) and arsenious sulphide (–ve sol) bring them in the precipitated forms. This type of coagulation is called mutual coagulation.
(c) By boiling : When a sol is boiled, the adsorbed layer is disturbed due to increased collisions with the molecules of dispersion medium. This reduces the charge on the particles and ultimately lead to settling down in the form of a precipitate.
(d) By persistent dialysis : On prolonged dialysis, traces of the electrolyte present in the sol are removed almost completely and the colloids become unstable and ultimately coagulate.
(e) By addition of electrolytes : When excess of an electrolyte is added, the colloidal particles are precipitated. The reason is that colloids interact with ions carrying charge opposite to that present on themselves. This causes neutralisation leading to their coagulation. The ion responsible for neutralisation of charge on the particles is called the coagulating ion. A negative ion causes the precipitation of positively charged sol and vice versa.
● It has been observed that, generally, the greater the valence of the flocculating ion added, the greater is its power to cause precipitation. This is known as Hardy-Schulze rule.
● In the coagulation of a negative sol, the flocculating power is in the order : `Al^(3+) > Ba^(2+) > Na^+`.
● Similarly, in the coagulation of a positive sol, the flocculating power is in the order : `[Fe(CN)_6]^(4–) > PO_4^(3–) > SO_4^(2–) > Cl^–`
● The minimum concentration of an electrolyte in millimoles per litre required to cause precipitation of a sol in two hours is called coagulating value. The smaller the quantity needed, the higher will be the coagulating power of an ion.
(i) `text(Colligative Properties :)` Colloidal particles being bigger aggregates, the number of particles in a colloidal solution is comparatively small as compared to a true solution. Hence, the values of colligative properties (osmotic pressure, lowering in vapour pressure, depression in freezing point and elevation in boiling point) are of small order as compared to values shown by true solutions at same concentrations.
(ii) `text(Tyndall Effect :)`
● If a homogeneous solution placed in dark is observed in the direction of light, it appears clear and, if it is observed from a direction at right angles to the direction of light beam, it appears perfectly dark.
● Colloidal solutions viewed in the same way may also appear reasonably clear or translucent by the transmitted light but they show a mild to strong opalescence, when viewed at right angles to the passage of light, i.e., the path of the beam is illuminated by a bluish light.
● This effect was first observed by Faraday and later studied in detail by Tyndall and is termed as Tyndall effect.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
Tyndall effect may be defined as the scattering of light by colloidal particles present in a colloidal solution.
● The bright cone of the light is called Tyndall cone.
● The Tyndall effect is due to the fact that colloidal particles scatter light in all directions in space.
● This scattering of light illuminates the path of beam in the colloidal dispersion.
● Tyndall effect can be observed during the projection of picture in the cinema hall due to scattering of light by dust and smoke particles present there.
● Tyndall effect is observed only when the following two conditions are satisfied.
(a) The diameter of the dispersed particles is not much smaller than the wavelength of the light used; and
(b) The refractive indices of the dispersed phase and the dispersion medium differ greatly in magnitude.
● Tyndall effect is used to distinguish between a colloidal and true solution.
● Zsigmondy, in 1903, used Tyndall effect to set up an apparatus known as ultramicroscope.
● An intense beam of light is focused on the colloidal solution contained in a glass vessel.
● The focus of the light is then observed with a microscope at right angles to the beam.
● Individual colloidal particles appear as bright stars against a dark background.
● Ultramicroscope does not render the actual colloidal particles visible but only observe the light scattered by them.
● Thus, ultramicroscope does not provide any information about the size and shape of colloidal particles.
(iii) `text(Colour :)`
● The colour of colloidal solution depends on the wavelength of light scattered by the dispersed particles.
● The wavelength of light further depends on the size and nature of the particles.
● The colour of colloidal solution also changes with the manner in which the observer receives the light.
● Example : A mixture of milk and water appears blue when viewed by the reflected light and red when viewed by the transmitted light. Finest gold sol is red in colour; as the size of particles increases, it appears purple, then blue and finally golden.
(iv) `text(Brownian Movement :)`
● When colloidal solutions are viewed under a powerful ultramicroscope, the colloidal particles appear to be in a state of continuous zig-zag motion all over the field of view.
● This motion was first observed by the British botanist, Robert Brown, and is known as Brownian movement.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
Brownian movement may be described as continuous zig-zag movement of the colloidal particles in a colloidal sol.
● This motion is independent of the nature of the colloid but depends on the size of the particles and viscosity of the solution.
● Smaller the size and lesser the viscosity, faster is the motion.
● The Brownian movement has been explained to be due to the unbalanced bombardment of the particles by the molecules of the dispersion medium.
● The Brownian movement has a stirring effect which does not permit the particles to settle and thus, is responsible for the stability of sols.
(v) `text(Charge on Colloidal Particles :)`
● Colloidal particles always carry an electric charge.
● The nature of this charge is the same on all the particles in a given colloidal solution and may be either positive or negative.
● A list of some common sols with the nature of charge on their particles is given in the Table.
● The charge on the sol particles is due to one or more reasons, viz., due to electron capture by sol particles during electrodispersion of metals, due to preferential adsorption of ions from solution and/or due to formulation of electrical double layer. Preferential adsorption of ions is the most accepted reason. The sol particles acquire positive or negative charge by preferential adsorption of `+ve` or `–ve` ions. When two or more ions are present in the dispersion medium, preferential adsorption of the ion common to the colloidal particle usually takes place. This can be explained by taking the following examples :
(a) When silver nitrate solution is added to potassium iodide solution, the precipitated silver iodide adsorbs iodide ions from the dispersion medium and negatively charged colloidal solution results. However, when `KI` solution is added to `AgNO_3` solution, positively charged sol results due to adsorption of `Ag^+` ions from dispersion medium.
`undersettext(Negatively charged)((AgI)//I^(-)) \ \ \ \ \ \ \ undersettext(Positively charged)((AgI)//(Ag^+))`
(b) If `FeCl_3` is added to excess of hot water, a positively charged sol of hydrated ferric oxide is formed due to adsorption of `Fe^(3+)` ions. However, when ferric chloride is added to `NaOH` a negatively charged sol is obtained with adsorption of `OH^-` ions.
`undersettext(Positively charged) (Fe_2O_3 . x H_2O //Fe^(3+)) \ \ \ \ undersettext(Negatively charged) (Fe_2O_3 . x H_2O //OH^-)`
● Having acquired a positive or a negative charge by selective adsorption on the surface of a colloidal particle as stated above, this layer attracts counter ions from the medium forming a second layer, as shown below.
`AgI//I^- K^+ \ \ \ \ \ \ \ \ \ AgI//Ag^+ I^-`
● The combination of the two layers of opposite charges around the colloidal particle is called Helmholtz electrical double layer.
● According to modern views, the first layer of ions is firmly held and is termed fixed layer while the second layer is mobile which is termed diffused layer.
● Since separation of charge is a seat of potential, the charges of opposite signs on the fixed and diffused parts of the double layer results in a difference in potential between these layers.
● This potential difference between the fixed layer and the diffused layer of opposite charges is called the electrokinetic potential or zeta potential.
● The presence of equal and similar charges on colloidal particles is largely responsible in providing stability to the colloidal solution, because the repulsive forces between charged particles having same charge prevent them from coalescing or aggregating when they come closer to one another.
(vi) `text(Electrophoresis :)` The existence of charge on colloidal particles is confirmed by electrophoresis experiment. When electric potential is applied across two platinum electrodes dipping in a colloidal solution, the colloidal particles move towards one or the other electrode.
● The movement of colloidal particles under an applied electric potential is called electrophoresis. Positively charged particles move towards the cathode while negatively charged particles move towards the anode.
● This can be demonstrated by the following experimental setup.
● When electrophoresis, i.e., movement of particles is prevented by some suitable means, it is observed that the dispersion medium begins to move in an electric field. This phenomenon is termed electroosmosis.
(vii) `text(Coagulation or Precipitation :)` The stability of the lyophobic sols is due to the presence of charge on colloidal particles. If, somehow, the charge is removed, the particles will come nearer to each other to form aggregates (or coagulate) and settle down under the force of gravity.
The process of settling of colloidal particles is called coagulation or precipitation of the sol. The coagulation of the lyophobic sols can be carried out in the following ways :
(a) By electrophoresis : The colloidal particles move towards oppositely charged electrodes, get discharged and precipitated.
(b) By mixing two oppositely charged sols : Oppositely charged sols when mixed in almost equal proportions, neutralise their charges and get partially or completely precipitated. Mixing of hydrated ferric oxide (+ve sol) and arsenious sulphide (–ve sol) bring them in the precipitated forms. This type of coagulation is called mutual coagulation.
(c) By boiling : When a sol is boiled, the adsorbed layer is disturbed due to increased collisions with the molecules of dispersion medium. This reduces the charge on the particles and ultimately lead to settling down in the form of a precipitate.
(d) By persistent dialysis : On prolonged dialysis, traces of the electrolyte present in the sol are removed almost completely and the colloids become unstable and ultimately coagulate.
(e) By addition of electrolytes : When excess of an electrolyte is added, the colloidal particles are precipitated. The reason is that colloids interact with ions carrying charge opposite to that present on themselves. This causes neutralisation leading to their coagulation. The ion responsible for neutralisation of charge on the particles is called the coagulating ion. A negative ion causes the precipitation of positively charged sol and vice versa.
● It has been observed that, generally, the greater the valence of the flocculating ion added, the greater is its power to cause precipitation. This is known as Hardy-Schulze rule.
● In the coagulation of a negative sol, the flocculating power is in the order : `Al^(3+) > Ba^(2+) > Na^+`.
● Similarly, in the coagulation of a positive sol, the flocculating power is in the order : `[Fe(CN)_6]^(4–) > PO_4^(3–) > SO_4^(2–) > Cl^–`
● The minimum concentration of an electrolyte in millimoles per litre required to cause precipitation of a sol in two hours is called coagulating value. The smaller the quantity needed, the higher will be the coagulating power of an ion.