Emulsions :
`=>` These are liquid-liquid colloidal systems, i.e., the dispersion of finely divided droplets in another liquid.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
An emulsion is a colloidal dispersion in which both the dispersed phase and dispersion medium are liquids.
`=>` If a mixture of two immiscible or partially miscible liquids is shaken, a coarse dispersion of one liquid in the other is obtained which is called emulsion.
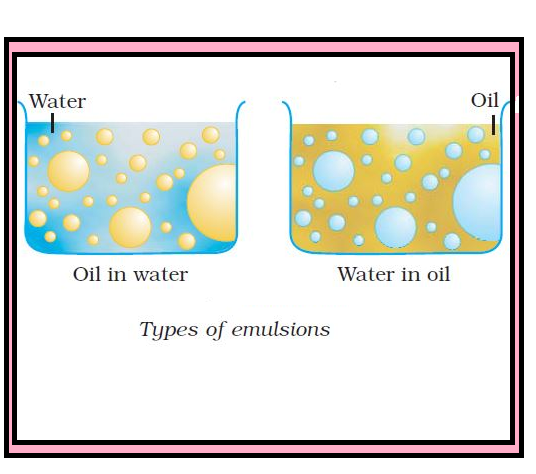
`=>` Generally, one of the two liquids is water. There are two types of emulsions :
(i) Oil dispersed in water (O/W type) : In this, water acts as dispersion medium. Examples of this type of emulsion are milk and vanishing cream. In milk, liquid fat is dispersed in water. Emulsions of oil in water are unstable and sometimes they separate into two layers on standing.
(ii) Water dispersed in oil (W/O type) : In this, oil acts as dispersion medium. Common examples of this type are butter and cream.
`text(Emulsifying Agent :)` For stabilisation of an emulsion, a third component called emulsifying agent is usually added. The emulsifying agent forms an interfacial film between suspended particles and the medium.
● The principal emulsifying agents for O/W emulsions are proteins, gums, natural and synthetic soaps, etc.
● For W/O, heavy metal salts of fatty acids, long chain alcohols, lampblack, etc.
● Emulsions can be diluted with any amount of the dispersion medium.
● On the other hand, the dispersed liquid when mixed, forms a separate layer. The droplets in emulsions are often negatively charged and can be precipitated by electrolytes.
● They also show Brownian movement and Tyndall effect.
● Emulsions can be broken into constituent liquids by heating, freezing, centrifuging, etc.
`text(Properties of emulsions)`:
•They exhibit all properties like tyndall effect, Brownian movement.
•Coagulation on addition of electrolyte.
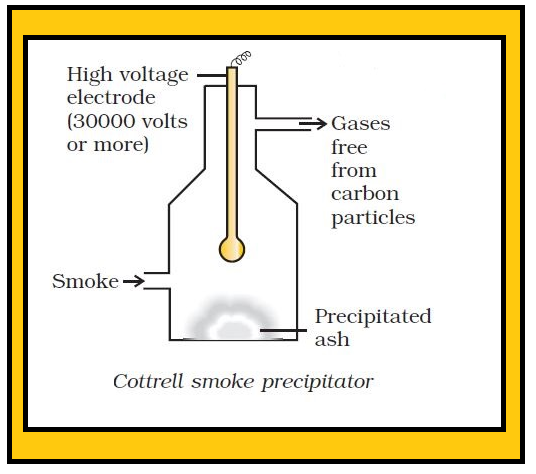
•Can be separated into their constituents liquids by boiling, freezing, centrifuging, electrostatic precipitation etc. The separation of cream from milk is a well known example of centrifuging.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
An emulsion is a colloidal dispersion in which both the dispersed phase and dispersion medium are liquids.
`=>` If a mixture of two immiscible or partially miscible liquids is shaken, a coarse dispersion of one liquid in the other is obtained which is called emulsion.
`=>` Generally, one of the two liquids is water. There are two types of emulsions :
(i) Oil dispersed in water (O/W type) : In this, water acts as dispersion medium. Examples of this type of emulsion are milk and vanishing cream. In milk, liquid fat is dispersed in water. Emulsions of oil in water are unstable and sometimes they separate into two layers on standing.
(ii) Water dispersed in oil (W/O type) : In this, oil acts as dispersion medium. Common examples of this type are butter and cream.
`text(Emulsifying Agent :)` For stabilisation of an emulsion, a third component called emulsifying agent is usually added. The emulsifying agent forms an interfacial film between suspended particles and the medium.
● The principal emulsifying agents for O/W emulsions are proteins, gums, natural and synthetic soaps, etc.
● For W/O, heavy metal salts of fatty acids, long chain alcohols, lampblack, etc.
● Emulsions can be diluted with any amount of the dispersion medium.
● On the other hand, the dispersed liquid when mixed, forms a separate layer. The droplets in emulsions are often negatively charged and can be precipitated by electrolytes.
● They also show Brownian movement and Tyndall effect.
● Emulsions can be broken into constituent liquids by heating, freezing, centrifuging, etc.
`text(Properties of emulsions)`:
•They exhibit all properties like tyndall effect, Brownian movement.
•Coagulation on addition of electrolyte.
•Can be separated into their constituents liquids by boiling, freezing, centrifuging, electrostatic precipitation etc. The separation of cream from milk is a well known example of centrifuging.