Extraction of Aluminium from alumina :
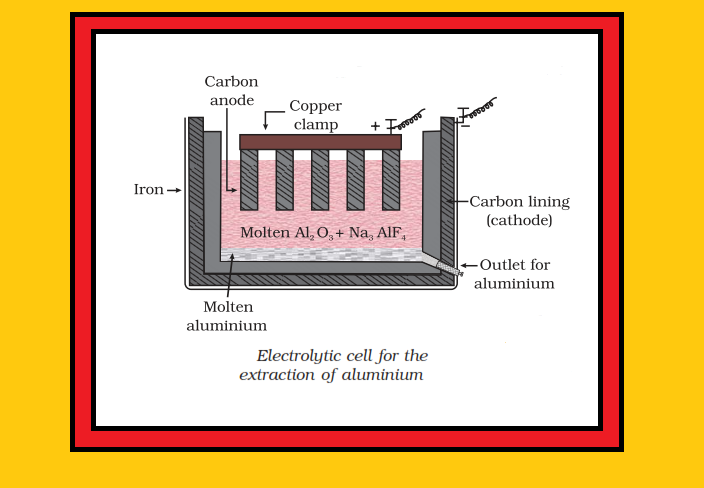
`=>` In the metallurgy of aluminium, purified `Al_2O_3` is mixed with `Na_3AlF_6` or `CaF_2` which lowers the melting point of the mix and brings conductivity.
`=>` The fused matrix is electrolysed.
● Cathode : Steel
● Anode : Graphite
`=>` The graphite anode is useful here for reduction to the metal.
`=>` The overall reaction may be taken as :
`2Al_2O_3 +3C → 4Al +3CO_2` .........(44)
`=>` This process of electrolysis is widely known as `text(Hall-Heroult)` process.
`=>` The electrolysis of the molten mass is carried out in an electrolytic cell using carbon electrodes.
`=>` The oxygen liberated at anode reacts with the carbon of anode producing `CO` and `CO_2`. This way for each kg of aluminium produced, about 0.5 kg of carbon anode is burnt away.
`=>` The electrolytic reactions are :
Cathode : `Al^(3+)` (melt) `+3 e^(-) → Al (l)` .......(45)
Anode : `C(s) + O^(2-) ` (melt) `→ CO (g) + 2e^(-)` ......(46)
`C(s) +2O^(2-) ` (melt) `→ CO_2 (g) +4e^(-)` .........(47)
`=>` The fused matrix is electrolysed.
● Cathode : Steel
● Anode : Graphite
`=>` The graphite anode is useful here for reduction to the metal.
`=>` The overall reaction may be taken as :
`2Al_2O_3 +3C → 4Al +3CO_2` .........(44)
`=>` This process of electrolysis is widely known as `text(Hall-Heroult)` process.
`=>` The electrolysis of the molten mass is carried out in an electrolytic cell using carbon electrodes.
`=>` The oxygen liberated at anode reacts with the carbon of anode producing `CO` and `CO_2`. This way for each kg of aluminium produced, about 0.5 kg of carbon anode is burnt away.
`=>` The electrolytic reactions are :
Cathode : `Al^(3+)` (melt) `+3 e^(-) → Al (l)` .......(45)
Anode : `C(s) + O^(2-) ` (melt) `→ CO (g) + 2e^(-)` ......(46)
`C(s) +2O^(2-) ` (melt) `→ CO_2 (g) +4e^(-)` .........(47)