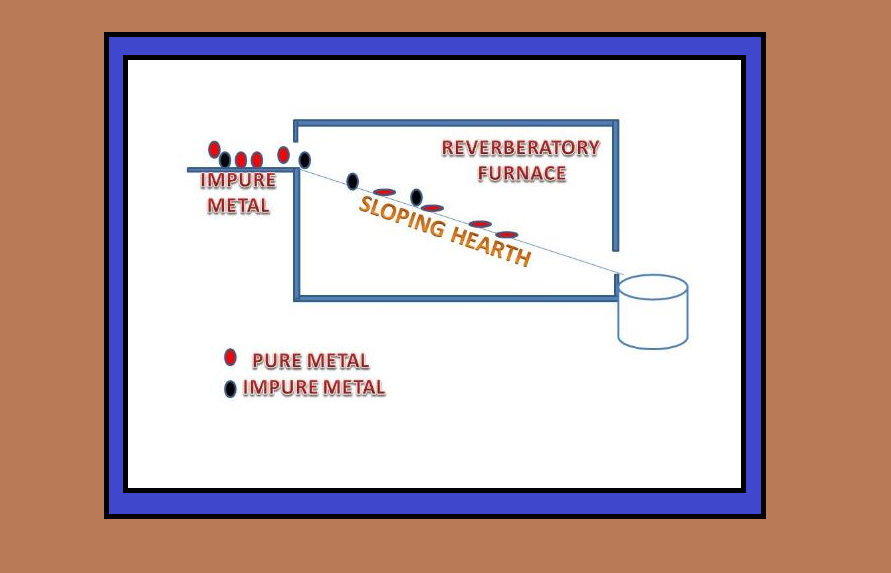
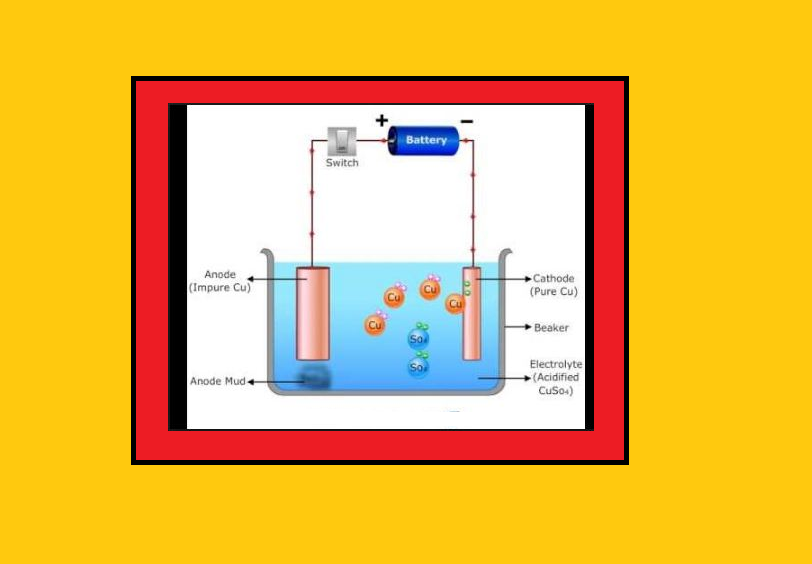
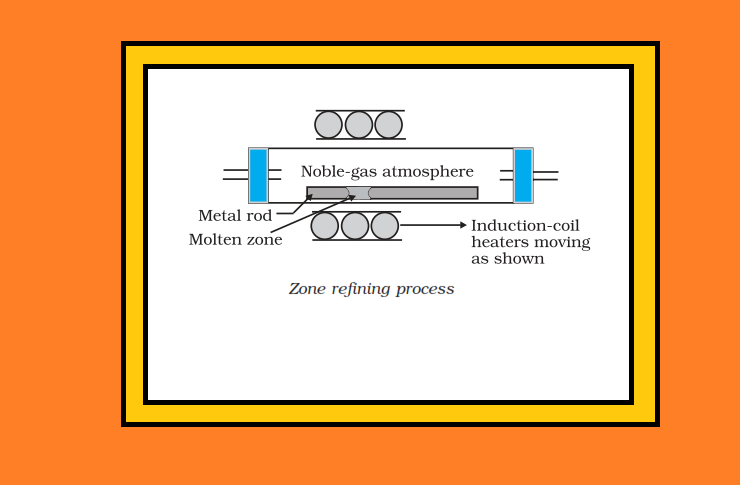
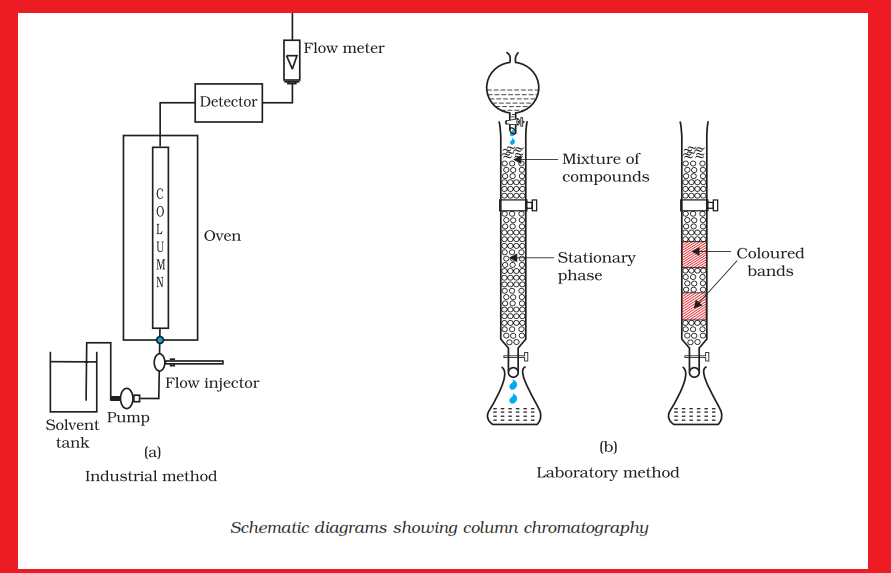
`text(Uses of Aluminium :)`
● Aluminium foils are used as wrappers for chocolates.
● The fine dust of the metal is used in paints and lacquers.
● Aluminium, being highly reactive, is also used in the extraction of chromium and manganese from their oxides.
● Wires of aluminium are used as electricity conductors.
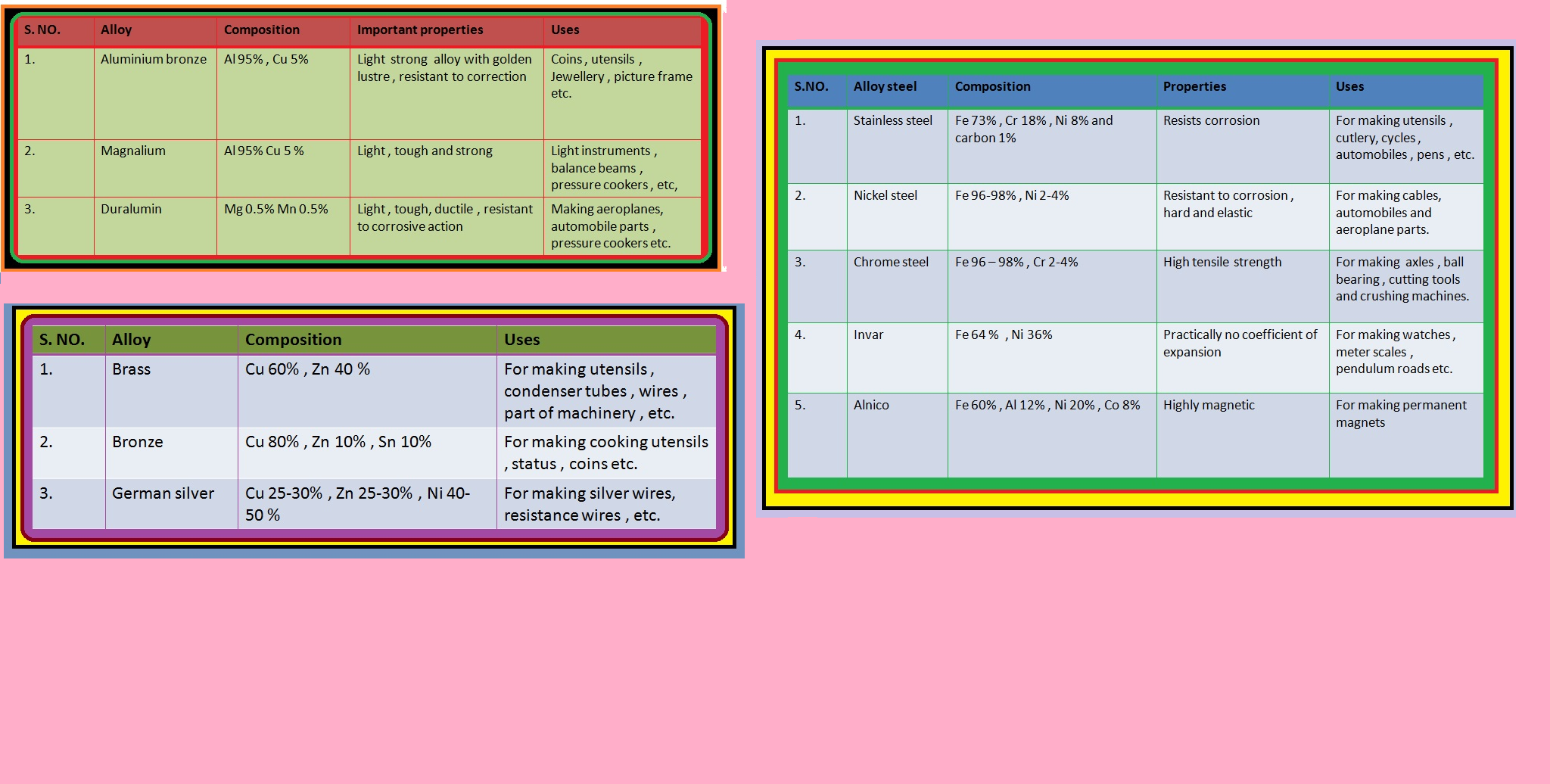
● Alloys containing aluminium, being light, are very useful.
`text(Uses of Copper :)`
● Copper is used for making wires used in electrical industry and for water and steam pipes.
● It is also used in several alloys that are rather tougher than the metal itself, e.g., brass (with zinc), bronze (with tin) and coinage alloy (with nickel).
`text(Uses of Zinc :)`
● Zinc is used for galvanising iron.
● It is also used in large quantities in batteries, as a constituent of many alloys, e.g., brass, (Cu 60%, Zn 40%) and german silver (Cu 25-30%, Zn 25-30%, Ni 40–50%).
● Zinc dust is used as a reducing agent in the manufacture of dye-stuffs, paints, etc.
`text(Uses of Iron :)`
● Cast iron, which is the most important form of iron, is used for casting stoves, railway sleepers, gutter pipes , toys, etc.
● It is used in the manufacture of wrought iron and steel.
● Wrought iron is used in making anchors, wires, bolts, chains and agricultural implements.
● Steel finds a number of uses. Alloy steel is obtained when other metals are added to it.
`->` Nickel steel is used for making cables, automobiles and aeroplane parts, pendulum, measuring tapes, chrome steel for cutting tools and crushing machines, and stainless steel for cycles, automobiles, utensils, pens, etc.
`text(Uses of Aluminium :)`
● Aluminium foils are used as wrappers for chocolates.
● The fine dust of the metal is used in paints and lacquers.
● Aluminium, being highly reactive, is also used in the extraction of chromium and manganese from their oxides.
● Wires of aluminium are used as electricity conductors.
● Alloys containing aluminium, being light, are very useful.
`text(Uses of Copper :)`
● Copper is used for making wires used in electrical industry and for water and steam pipes.
● It is also used in several alloys that are rather tougher than the metal itself, e.g., brass (with zinc), bronze (with tin) and coinage alloy (with nickel).
`text(Uses of Zinc :)`
● Zinc is used for galvanising iron.
● It is also used in large quantities in batteries, as a constituent of many alloys, e.g., brass, (Cu 60%, Zn 40%) and german silver (Cu 25-30%, Zn 25-30%, Ni 40–50%).
● Zinc dust is used as a reducing agent in the manufacture of dye-stuffs, paints, etc.
`text(Uses of Iron :)`
● Cast iron, which is the most important form of iron, is used for casting stoves, railway sleepers, gutter pipes , toys, etc.
● It is used in the manufacture of wrought iron and steel.
● Wrought iron is used in making anchors, wires, bolts, chains and agricultural implements.
● Steel finds a number of uses. Alloy steel is obtained when other metals are added to it.
`->` Nickel steel is used for making cables, automobiles and aeroplane parts, pendulum, measuring tapes, chrome steel for cutting tools and crushing machines, and stainless steel for cycles, automobiles, utensils, pens, etc.