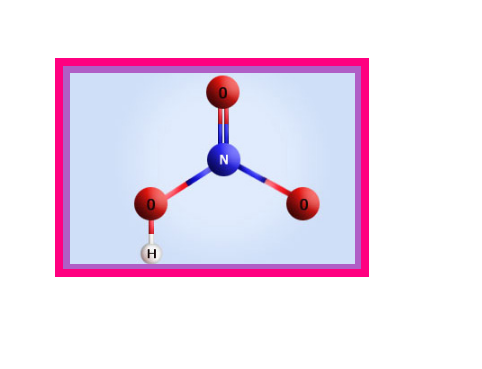
Oxides of Nitrogen :
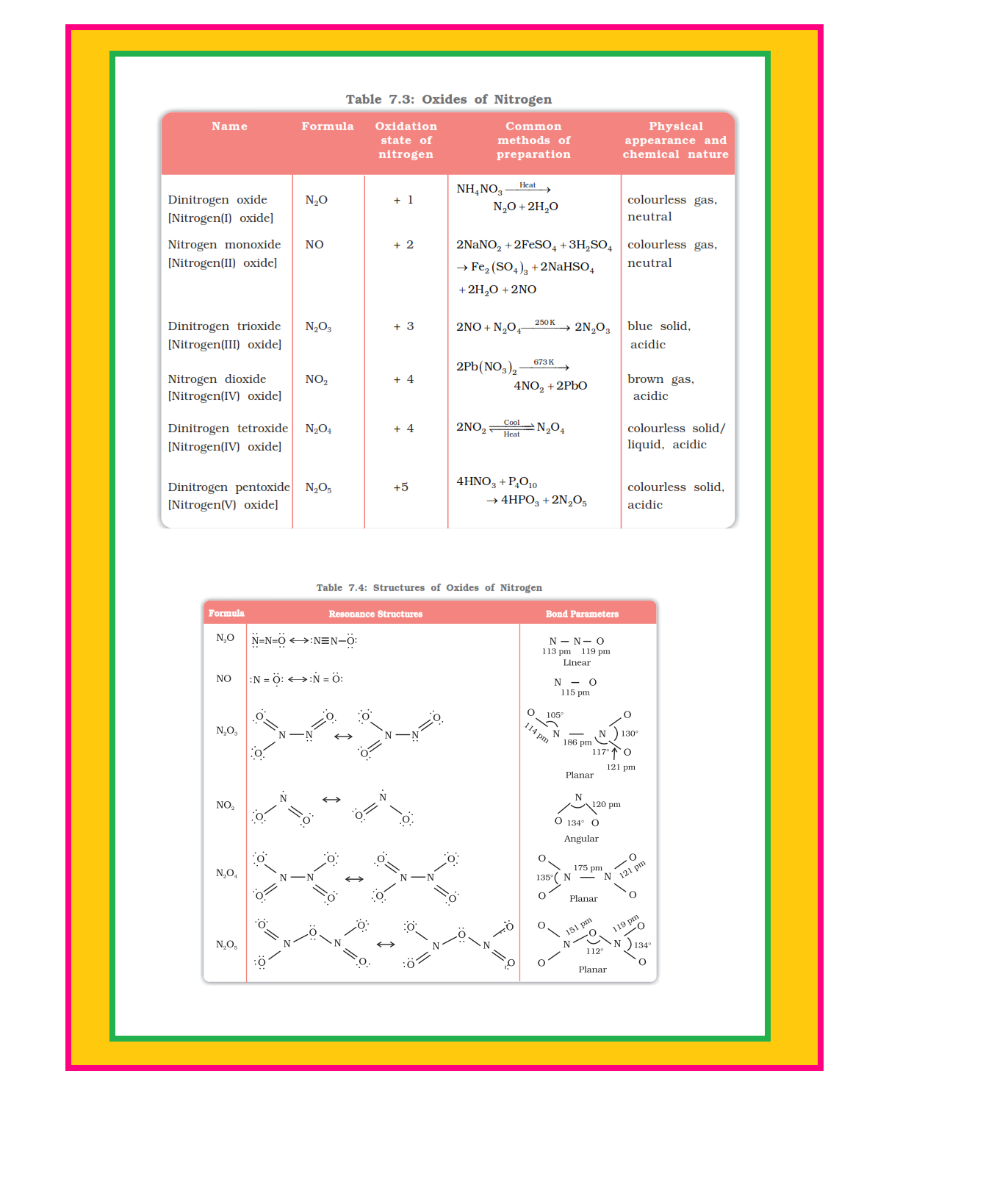
Nitrogen forms a number of oxides in different oxidation states. The names, formulas, preparation and physical appearance of these oxides are given in Table 7.3.
Lewis dot main resonance structures and bond parameters of oxides are given in Table 7.4.
Lewis dot main resonance structures and bond parameters of oxides are given in Table 7.4.