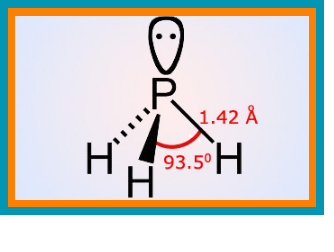
White phosphorus :
● It is a translucent white waxy solid.
● It is poisonous, insoluble in water but soluble in carbon disulphide
● It glows in dark (chemiluminescence).
● It dissolves in boiling `color{red}(NaOH)` solution in an inert atmosphere giving `color{red}(PH_3)`.
`color{red}(P_4+3NaOH +3H_2O → PH_3 + undersettext{(sodium hypophosphite)}(3NaH_2PO_2))`
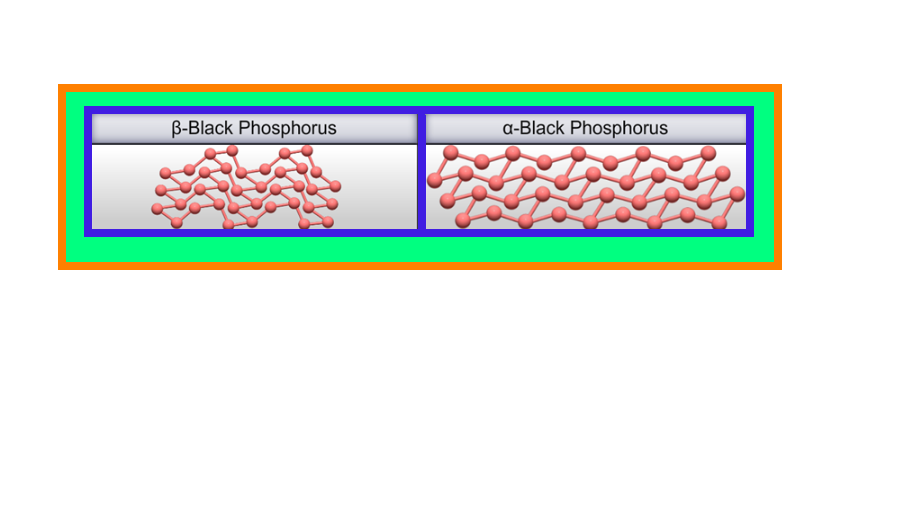
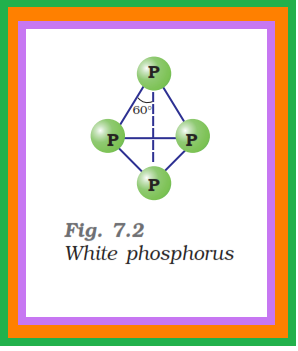
● White phosphorus is less stable and therefore, more reactive than the other solid phases under normal conditions because of angular
strain in the `color{red}(P_4)` molecule where the angles are only `60^0`.
● It readily catches fire in air to give dense white fumes of `color{red}(P_4O_(10)).`
`color{red}(P_4+5O_2 → P_4O_(10))`
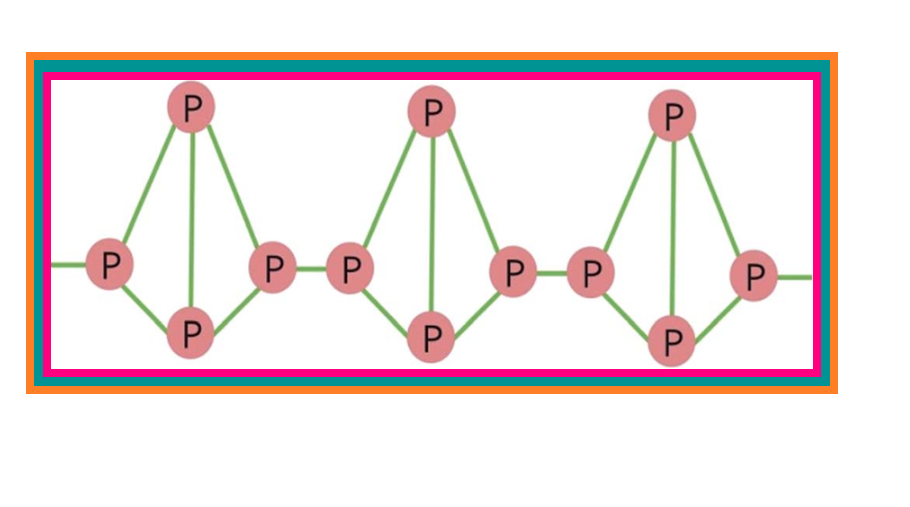
● It consists of discrete tetrahedral `color{red}(P_4)` molecule as shown in Fig. 7.2.
● It is poisonous, insoluble in water but soluble in carbon disulphide
● It glows in dark (chemiluminescence).
● It dissolves in boiling `color{red}(NaOH)` solution in an inert atmosphere giving `color{red}(PH_3)`.
`color{red}(P_4+3NaOH +3H_2O → PH_3 + undersettext{(sodium hypophosphite)}(3NaH_2PO_2))`
● White phosphorus is less stable and therefore, more reactive than the other solid phases under normal conditions because of angular
strain in the `color{red}(P_4)` molecule where the angles are only `60^0`.
● It readily catches fire in air to give dense white fumes of `color{red}(P_4O_(10)).`
`color{red}(P_4+5O_2 → P_4O_(10))`
● It consists of discrete tetrahedral `color{red}(P_4)` molecule as shown in Fig. 7.2.