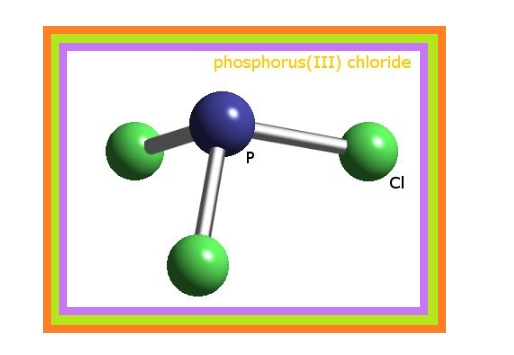
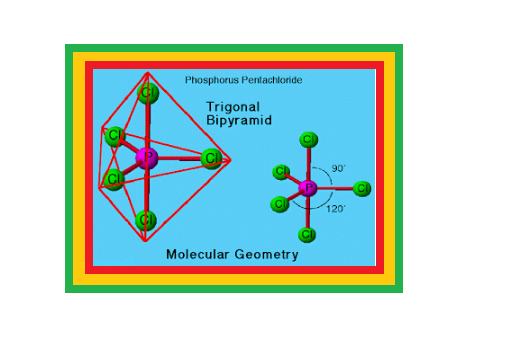
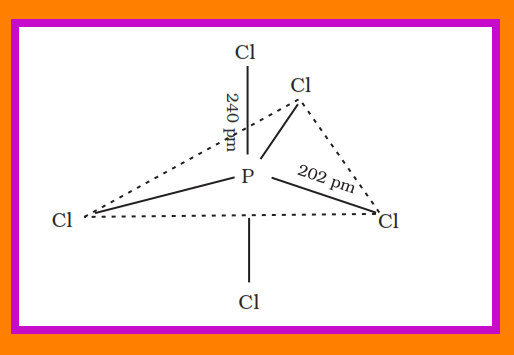
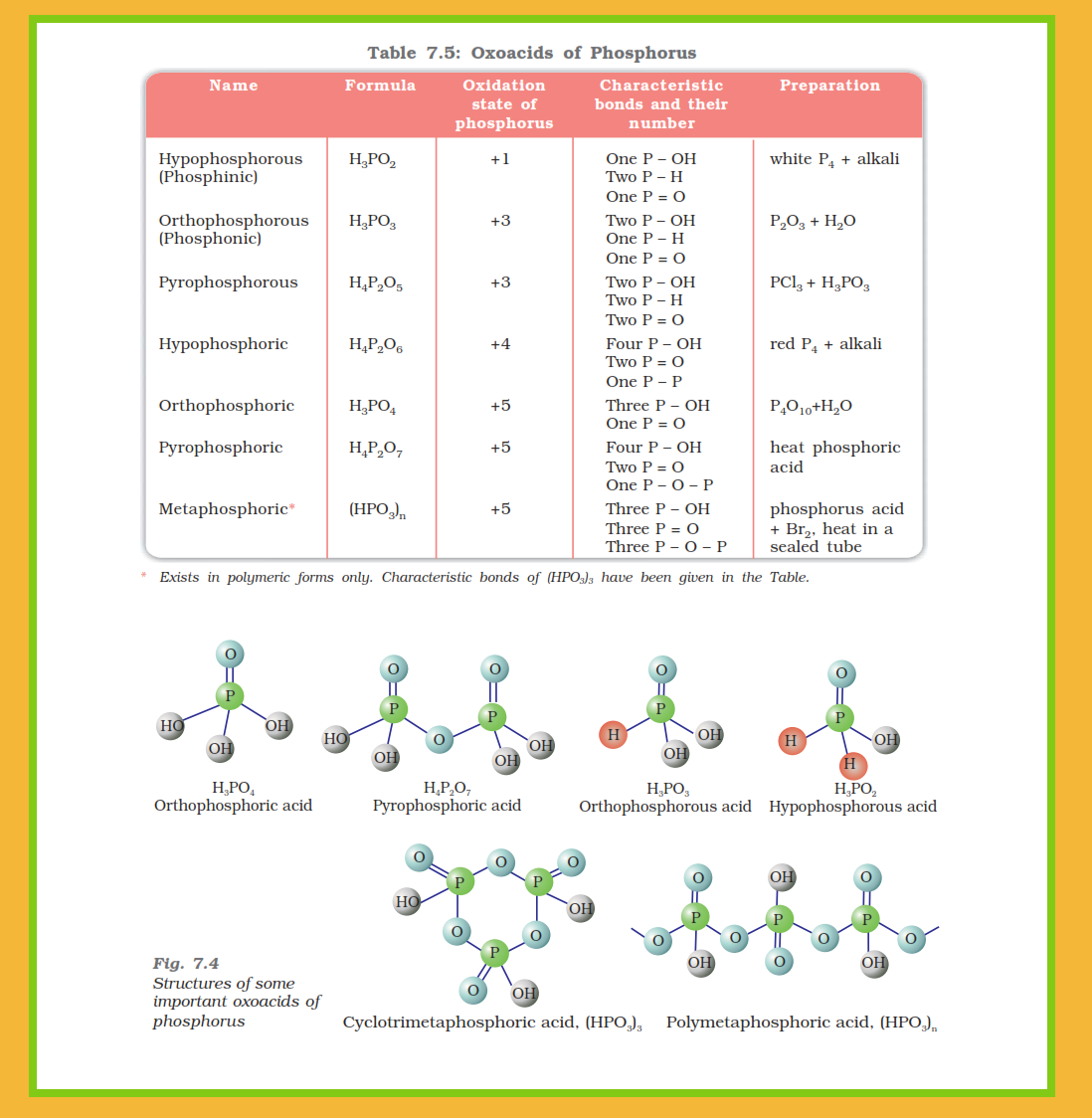
`=>` Phosphorus forms a number of oxoacids.
`=>` The important oxoacids of phosphorus with their formulas, methods of preparation and the presence of some characteristic bonds in their structures are given in Table 7.5.
● In oxoacids, phosphorus is tetrahedrally surrounded by other atoms.
● All these acids contain one `color{red}(P=O)` and at least one `color{red}(P–OH)` bond.
● The oxoacids in which phosphorus has lower oxidation state (less than `+5`) contain, in addition to `color{red}(P=O)` and `color{red}(P–OH)` bonds, either `color{red}(P–P)` (e.g., in `color{red}(H_4P_2O_6)`) or `color{red}(P–H)` (e.g., in `color{red}(H_3PO_2)`) bonds but not both.
● These acids in `+3` oxidation state of phosphorus tend to disproportionate to higher and lower oxidation states.
● `color{red}("Example")` : Orthophophorous acid (or phosphorous acid) on heating disproportionates to give orthophosphoric acid (or phosphoric acid) and phosphine.
`color{red}(4H_3PO_3 → 3H_3PO_4 +PH_3)`
● The acids which contain `P–H` bond have strong reducing properties. Thus, hypophosphorous acid is a good reducing agent as it contains two `color{red}(P–H)` bonds and reduces, for example, `color{red}(AgNO_3)` to metallic silver.
`color{red}(4AgNO_3+2H_2O +H_3PO_2 → 4Ag +4HNO_3+H_3PO_4)`
● The `color{red}(P–H)` bonds are not ionisable to give `color{red}(H^+)` and do not play any role in basicity.
● Only those `color{red}(H)` atoms which are attached with oxygen in `color{red}(P–OH)` form are ionisable and cause the basicity. Thus, `color{red}(H_3PO_3)` and `color{red}(H_3PO_4)` are dibasic and tribasic, respectively as the structure of `color{red}(H_3PO_3)` has two `color{red}(P–OH)` bonds and `color{red}(H_3PO_4)` three.
`=>` Phosphorus forms a number of oxoacids.
`=>` The important oxoacids of phosphorus with their formulas, methods of preparation and the presence of some characteristic bonds in their structures are given in Table 7.5.
● In oxoacids, phosphorus is tetrahedrally surrounded by other atoms.
● All these acids contain one `color{red}(P=O)` and at least one `color{red}(P–OH)` bond.
● The oxoacids in which phosphorus has lower oxidation state (less than `+5`) contain, in addition to `color{red}(P=O)` and `color{red}(P–OH)` bonds, either `color{red}(P–P)` (e.g., in `color{red}(H_4P_2O_6)`) or `color{red}(P–H)` (e.g., in `color{red}(H_3PO_2)`) bonds but not both.
● These acids in `+3` oxidation state of phosphorus tend to disproportionate to higher and lower oxidation states.
● `color{red}("Example")` : Orthophophorous acid (or phosphorous acid) on heating disproportionates to give orthophosphoric acid (or phosphoric acid) and phosphine.
`color{red}(4H_3PO_3 → 3H_3PO_4 +PH_3)`
● The acids which contain `P–H` bond have strong reducing properties. Thus, hypophosphorous acid is a good reducing agent as it contains two `color{red}(P–H)` bonds and reduces, for example, `color{red}(AgNO_3)` to metallic silver.
`color{red}(4AgNO_3+2H_2O +H_3PO_2 → 4Ag +4HNO_3+H_3PO_4)`
● The `color{red}(P–H)` bonds are not ionisable to give `color{red}(H^+)` and do not play any role in basicity.
● Only those `color{red}(H)` atoms which are attached with oxygen in `color{red}(P–OH)` form are ionisable and cause the basicity. Thus, `color{red}(H_3PO_3)` and `color{red}(H_3PO_4)` are dibasic and tribasic, respectively as the structure of `color{red}(H_3PO_3)` has two `color{red}(P–OH)` bonds and `color{red}(H_3PO_4)` three.