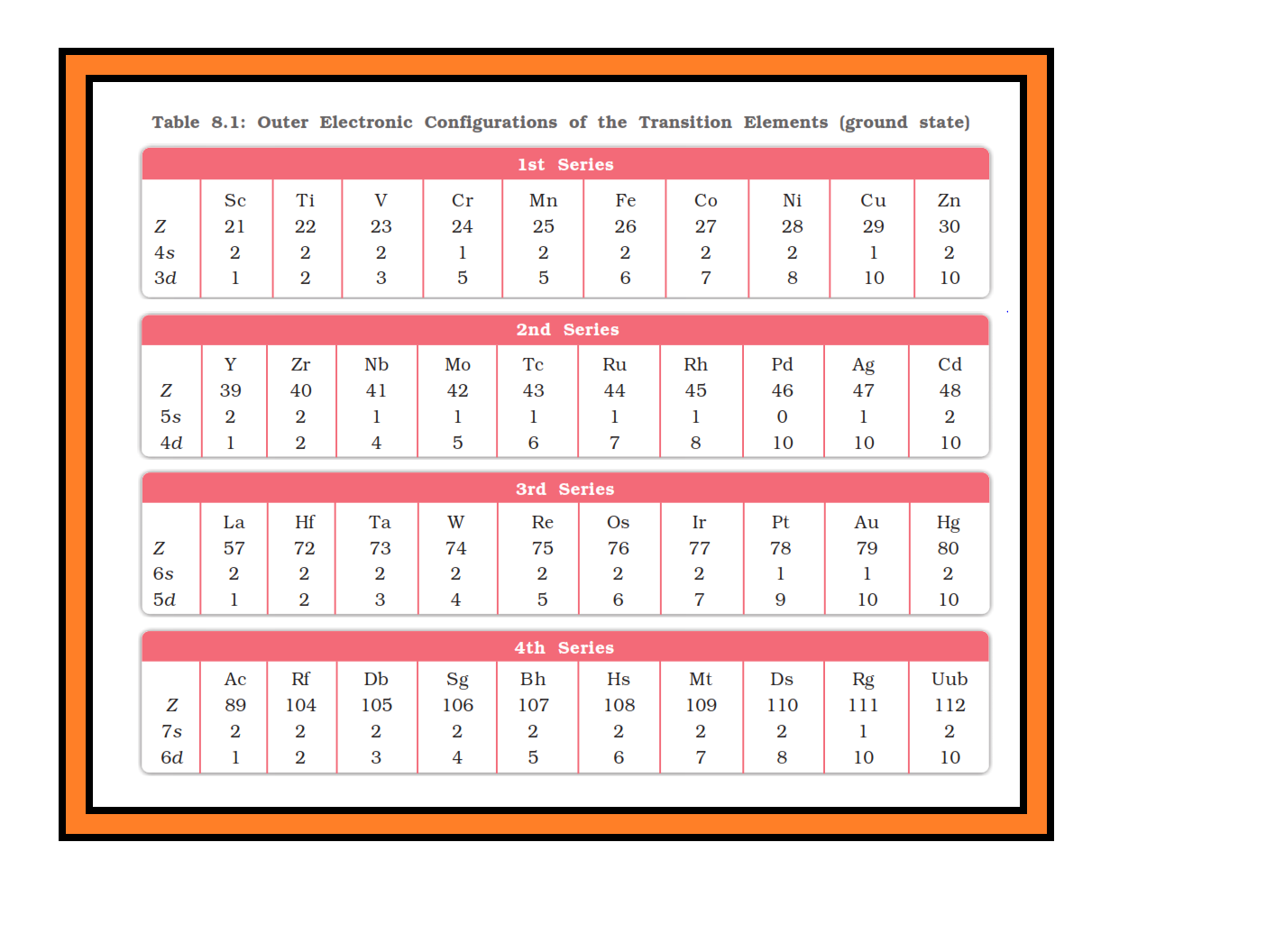
`=>` In general the electronic configuration of these elements is `color{red}((n-1)d^(1–10) ns^(1–2))`. The (`color{red}(n–1)`) stands for the inner `color{red}(d)`-orbitals which may have one to ten electrons and the outermost `color{red}(ns)`-orbital may have one or two electrons.
`=>` However, this generalisation has several exceptions because of very little energy difference between `color{red}((n-1)d)` and `color{red}(ns)` orbitals.
● Furthermore, half and completely filled sets of orbitals are relatively more stable.
● A result of this factor is reflected in the electronic configurations of `color{red}(Cr)` and `color{red}(Cu)` in the `color{red}(3d)` series.
● Consider the case of `color{red}(Cr)`, for example, which has `color{red}(3d^5 4s^1)` instead of `color{red}(3d^4 4s^2)`; the energy gap between the two sets (`color{red}(3d)` and `color{red}(4s)`) of orbitals is small enough to prevent electron entering the `color{red}(3d)` orbitals.
● Similarly in case of `color{red}(Cu)`, the configuration is `color{red}(3d^(10) 4s^1)` and not `color{red}(3d^9 4s^2)`. The outer electronic configurations of the transition elements are given in Table 8.1.
`=>` The electronic configurations of `color{red}(Zn)`, `color{red}(Cd)` and `color{red}(Hg)` are represented by the general formula `color{red}((n-1)d^(10) ns^2)`.
● The orbitals in these elements are completely filled in the ground state as well as in their common oxidation states.
● Therefore, they are not regarded as transition elements.
`=>` The `color{red}(d)`-orbitals of the transition elements project to the periphery of an atom more than the other orbitals (i.e., `color{red}(s)` and `color{red}(p)`), hence, they are more influenced by the surroundings as well as affecting the atoms or molecules surrounding them.
● In some respects, ions of a given `color{red}(d^n)` configuration (`color{red}(n = 1 - 9)`) have similar magnetic and electronic properties.
● With partly filled `color{red}(d)`-orbitals these elements exhibit certain characteristic properties such as display of a variety of oxidation states, formation of coloured ions and entering into complex formation with a variety of ligands.
● The transition metals and their compounds also exhibit catalytic property and paramagnetic behaviour.
`=>` There are greater horizontal similarities in the properties of the transition elements in contrast to the main group elements.
● However, some group similarities also exist.
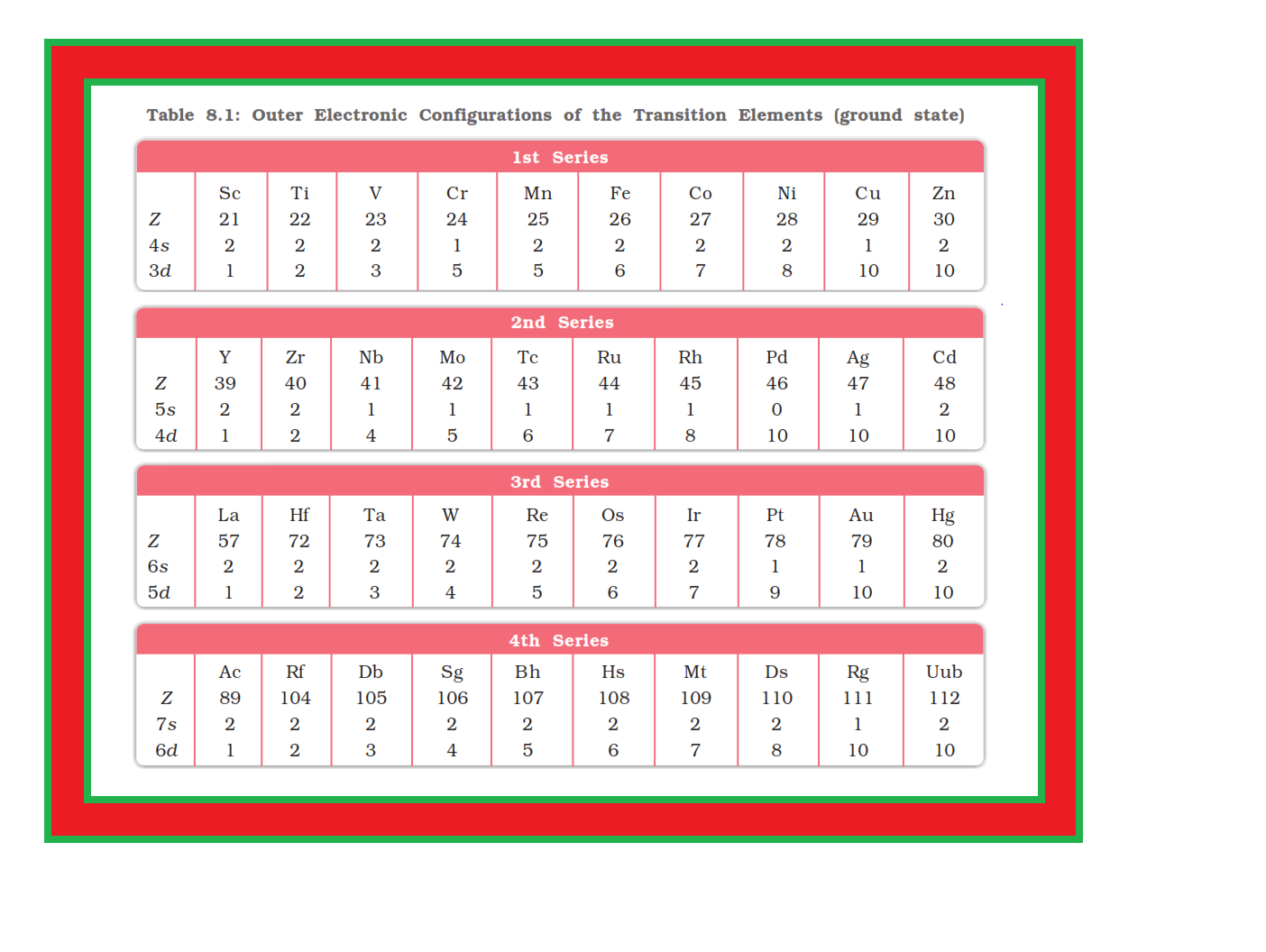
`=>` In general the electronic configuration of these elements is `color{red}((n-1)d^(1–10) ns^(1–2))`. The (`color{red}(n–1)`) stands for the inner `color{red}(d)`-orbitals which may have one to ten electrons and the outermost `color{red}(ns)`-orbital may have one or two electrons.
`=>` However, this generalisation has several exceptions because of very little energy difference between `color{red}((n-1)d)` and `color{red}(ns)` orbitals.
● Furthermore, half and completely filled sets of orbitals are relatively more stable.
● A result of this factor is reflected in the electronic configurations of `color{red}(Cr)` and `color{red}(Cu)` in the `color{red}(3d)` series.
● Consider the case of `color{red}(Cr)`, for example, which has `color{red}(3d^5 4s^1)` instead of `color{red}(3d^4 4s^2)`; the energy gap between the two sets (`color{red}(3d)` and `color{red}(4s)`) of orbitals is small enough to prevent electron entering the `color{red}(3d)` orbitals.
● Similarly in case of `color{red}(Cu)`, the configuration is `color{red}(3d^(10) 4s^1)` and not `color{red}(3d^9 4s^2)`. The outer electronic configurations of the transition elements are given in Table 8.1.
`=>` The electronic configurations of `color{red}(Zn)`, `color{red}(Cd)` and `color{red}(Hg)` are represented by the general formula `color{red}((n-1)d^(10) ns^2)`.
● The orbitals in these elements are completely filled in the ground state as well as in their common oxidation states.
● Therefore, they are not regarded as transition elements.
`=>` The `color{red}(d)`-orbitals of the transition elements project to the periphery of an atom more than the other orbitals (i.e., `color{red}(s)` and `color{red}(p)`), hence, they are more influenced by the surroundings as well as affecting the atoms or molecules surrounding them.
● In some respects, ions of a given `color{red}(d^n)` configuration (`color{red}(n = 1 - 9)`) have similar magnetic and electronic properties.
● With partly filled `color{red}(d)`-orbitals these elements exhibit certain characteristic properties such as display of a variety of oxidation states, formation of coloured ions and entering into complex formation with a variety of ligands.
● The transition metals and their compounds also exhibit catalytic property and paramagnetic behaviour.
`=>` There are greater horizontal similarities in the properties of the transition elements in contrast to the main group elements.
● However, some group similarities also exist.