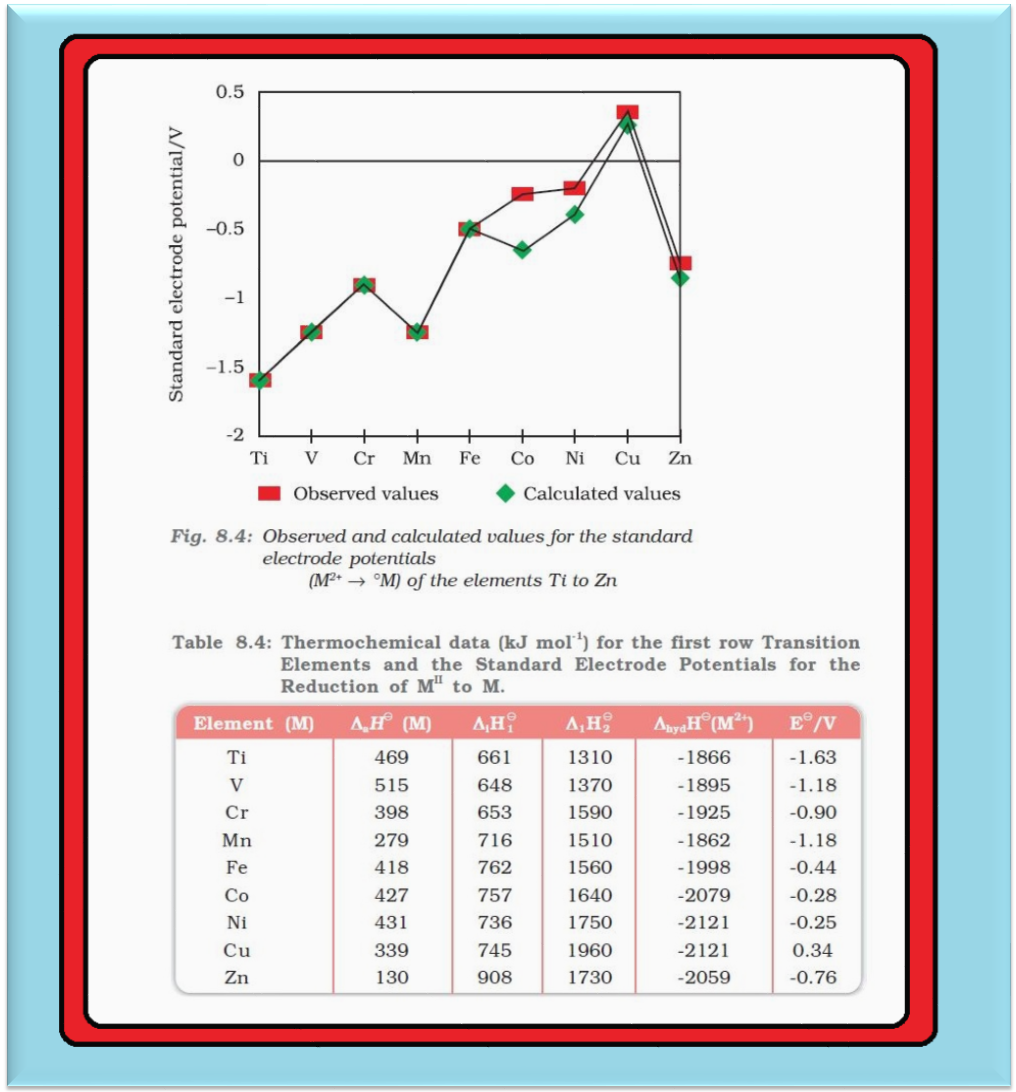
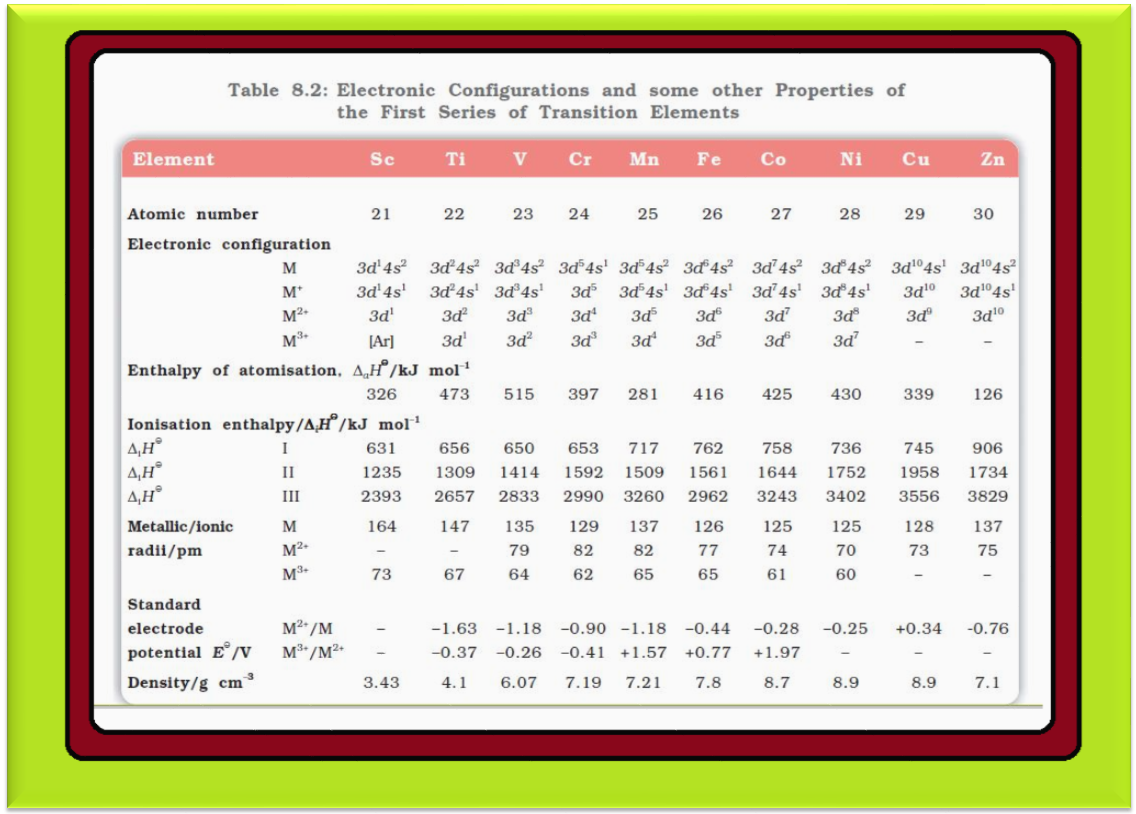
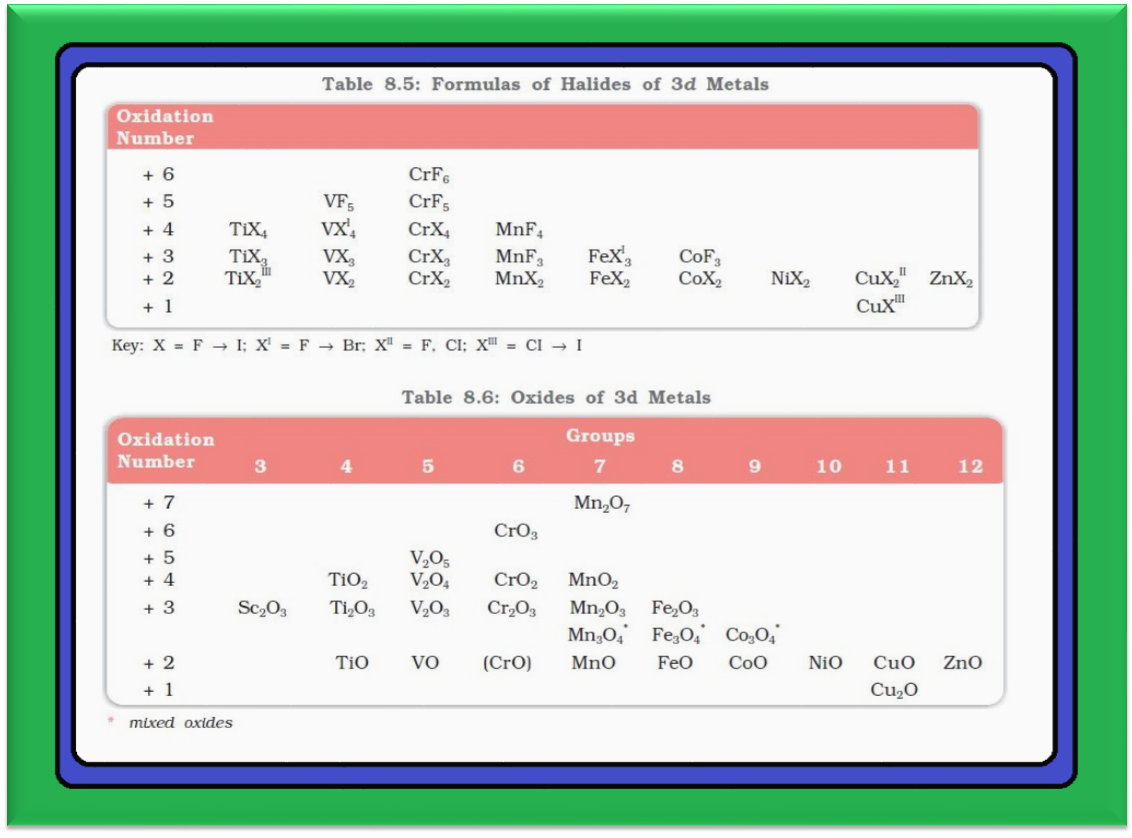
Oxidation States :
`=>` One of the notable features of a transition element is the great variety of oxidation states it may show in its compounds.
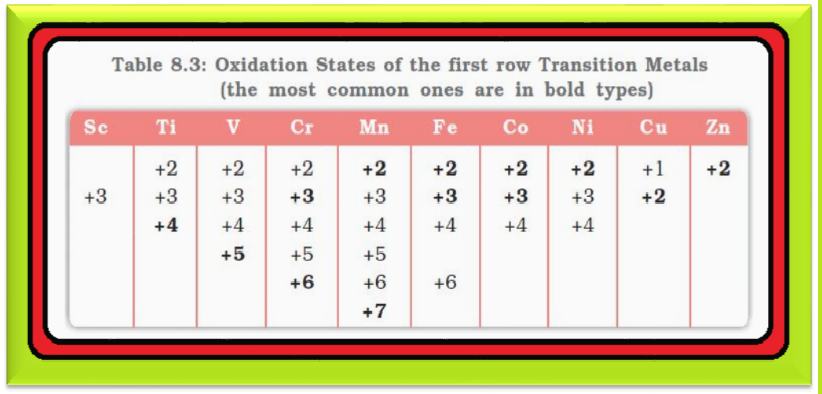
`=>` Table 8.3 lists the common oxidation states of the first row transition elements.
`=>` The elements which give the greatest number of oxidation states occur in or near the middle of the series.
● Manganese, for example, exhibits all the oxidation states from `+2` to `+7`.
`=>` The lesser number of oxidation states at the extreme ends stems from either too few electrons to lose or share (`color{red}(Sc)`, `color{red}(Ti)`) or too many `color{red}(d)` electrons (hence fewer orbitals available in which to share electrons with others) for higher valence (`color{red}(Cu)`, `color{red}(Zn)`).
● Thus, early in the series scandium(II) is virtually unknown and titanium (IV) is more stable than `color{red}(Ti )`(`III`) or `color{red}(Ti)`(`II`).
● At the other end, the only oxidation state of zinc is `+2` (no `color{red}(d)` electrons are involved).
● The maximum oxidation states of reasonable stability correspond in value to the sum of the `s` and `d` electrons upto manganese (`color{red}(Ti^(IV)O_2)`, `color{red}(V^(V)O_2^+)`, `color{red}(Cr^(VI)O_4^(2-))`, `color{red}(Mn^(VII)O_4^-)`) followed by a rather abrupt decrease in stability of higher oxidation states, so that the typical species to follow are `color{red}(Fe^(II,III))`, `color{red}(Co^(II,III))`, `color{red}(Ni^(II))`, `color{red}(Cu^(I,II))`, `color{red}(Zn^(II))`.
`=>` The variability of oxidation states, a characteristic of transition elements, arises out of incomplete filling of `d` orbitals in such a way that their oxidation states differ from each other by unity, e.g., `color{red}(V^(II))`, `color{red}(V^(III))`, `color{red}(V^(IV))`, `color{red}(V^V)`.
● This is not in agreement with the variability of oxidation states of non transition elements where oxidation states normally differ by a unit of two.
`=>` An interesting feature in the variability of oxidation states of the `color{red}(d)`–block elements is noticed among the groups (groups `4` through `10`).
`color{red}(text(Note )) : ` In the `color{red}(p)`–block the lower oxidation states are favoured by the heavier members (due to inert pair effect), the opposite is true in the groups of `color{red}(d)`-block.
● For example, in group 6, `color{red}(Mo(VI))` and `color{red}(W(VI))` are found to be more stable than `color{red}(Cr(VI))`. Thus `color{red}(Cr(VI))` in the form of dichromate in acidic medium is a strong oxidising agent, whereas `color{red}(MoO_3)` and `color{red}(WO_3)` are not.
`=>` Low oxidation states are found when a complex compound has ligands capable of `color{red}(π)`-acceptor character in addition to the `color{red}(σ)`-bonding. For example, in `color{red}(Ni(CO)_4)` and `color{red}(Fe(CO)_5)`, the oxidation state of nickel and iron is zero.
`=>` Table 8.3 lists the common oxidation states of the first row transition elements.
`=>` The elements which give the greatest number of oxidation states occur in or near the middle of the series.
● Manganese, for example, exhibits all the oxidation states from `+2` to `+7`.
`=>` The lesser number of oxidation states at the extreme ends stems from either too few electrons to lose or share (`color{red}(Sc)`, `color{red}(Ti)`) or too many `color{red}(d)` electrons (hence fewer orbitals available in which to share electrons with others) for higher valence (`color{red}(Cu)`, `color{red}(Zn)`).
● Thus, early in the series scandium(II) is virtually unknown and titanium (IV) is more stable than `color{red}(Ti )`(`III`) or `color{red}(Ti)`(`II`).
● At the other end, the only oxidation state of zinc is `+2` (no `color{red}(d)` electrons are involved).
● The maximum oxidation states of reasonable stability correspond in value to the sum of the `s` and `d` electrons upto manganese (`color{red}(Ti^(IV)O_2)`, `color{red}(V^(V)O_2^+)`, `color{red}(Cr^(VI)O_4^(2-))`, `color{red}(Mn^(VII)O_4^-)`) followed by a rather abrupt decrease in stability of higher oxidation states, so that the typical species to follow are `color{red}(Fe^(II,III))`, `color{red}(Co^(II,III))`, `color{red}(Ni^(II))`, `color{red}(Cu^(I,II))`, `color{red}(Zn^(II))`.
`=>` The variability of oxidation states, a characteristic of transition elements, arises out of incomplete filling of `d` orbitals in such a way that their oxidation states differ from each other by unity, e.g., `color{red}(V^(II))`, `color{red}(V^(III))`, `color{red}(V^(IV))`, `color{red}(V^V)`.
● This is not in agreement with the variability of oxidation states of non transition elements where oxidation states normally differ by a unit of two.
`=>` An interesting feature in the variability of oxidation states of the `color{red}(d)`–block elements is noticed among the groups (groups `4` through `10`).
`color{red}(text(Note )) : ` In the `color{red}(p)`–block the lower oxidation states are favoured by the heavier members (due to inert pair effect), the opposite is true in the groups of `color{red}(d)`-block.
● For example, in group 6, `color{red}(Mo(VI))` and `color{red}(W(VI))` are found to be more stable than `color{red}(Cr(VI))`. Thus `color{red}(Cr(VI))` in the form of dichromate in acidic medium is a strong oxidising agent, whereas `color{red}(MoO_3)` and `color{red}(WO_3)` are not.
`=>` Low oxidation states are found when a complex compound has ligands capable of `color{red}(π)`-acceptor character in addition to the `color{red}(σ)`-bonding. For example, in `color{red}(Ni(CO)_4)` and `color{red}(Fe(CO)_5)`, the oxidation state of nickel and iron is zero.