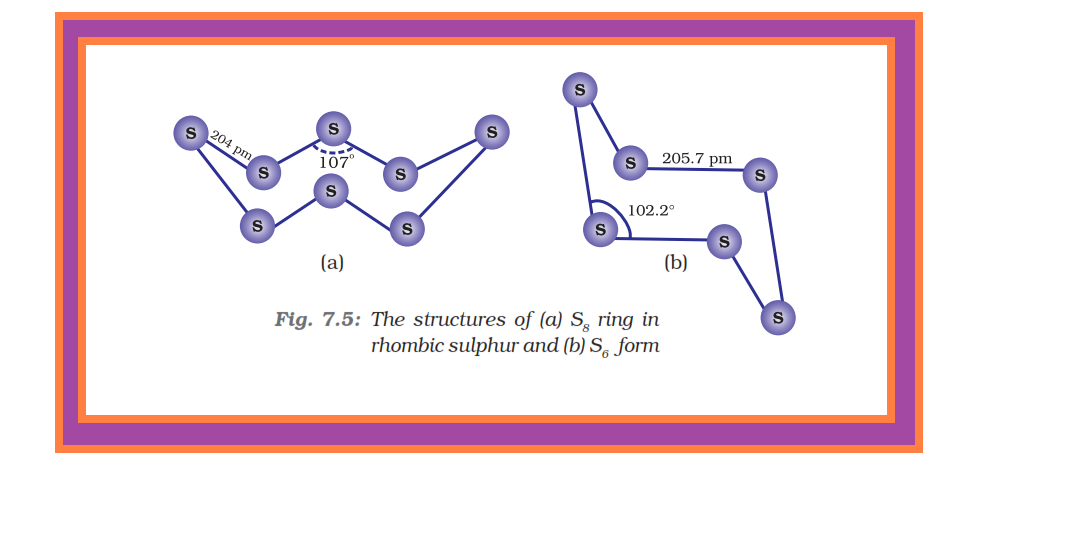
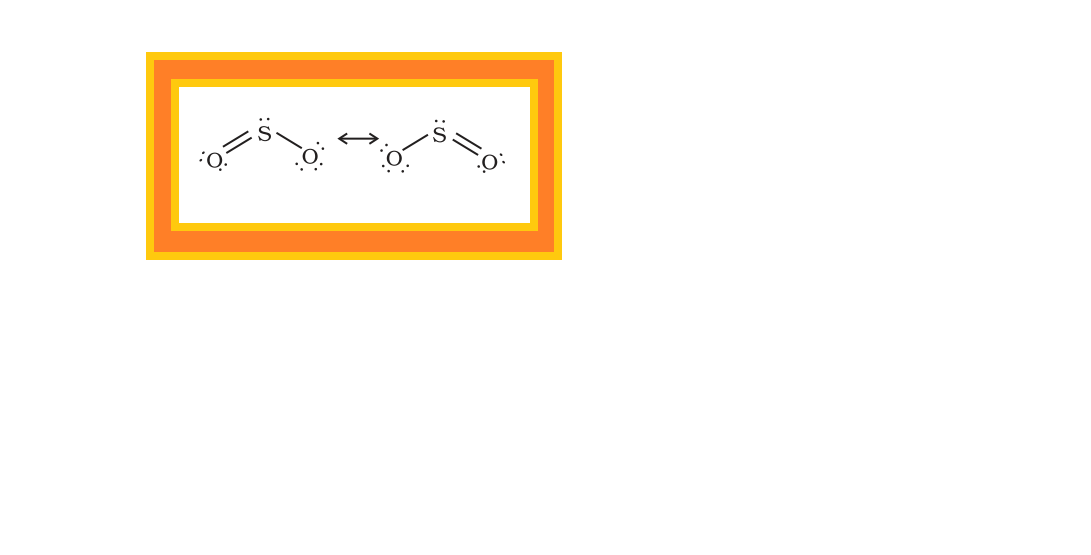
Sulphur — Allotropic Forms :
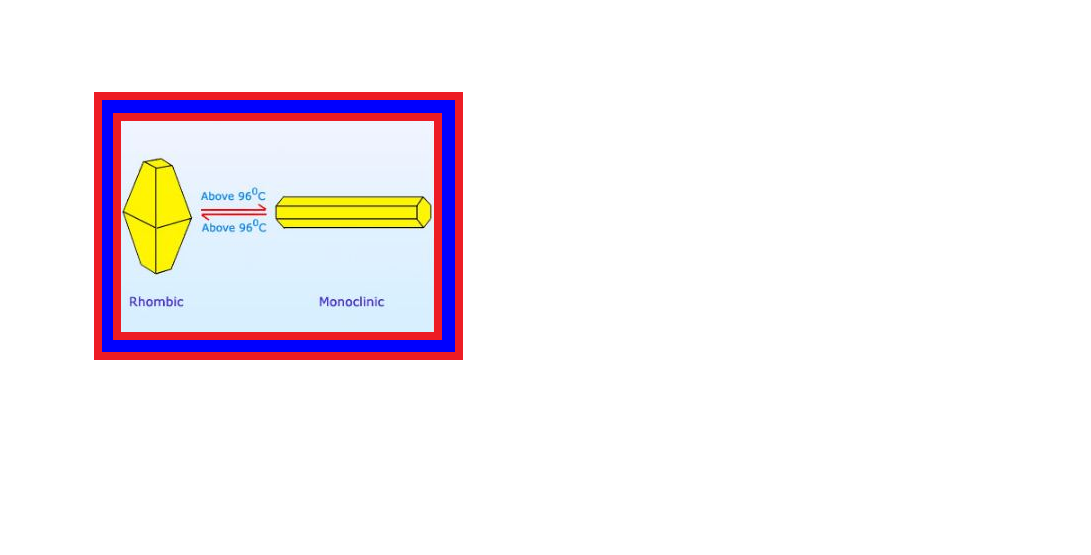
`=>` Sulphur forms numerous allotropes of which the yellow rhombic (`color{red}(α)`-sulphur) and monoclinic (`color{red}(β)`-sulphur) forms are the most important.
`=>` The stable form at room temperature is rhombic sulphur, which transforms to monoclinic sulphur when heated above `369 K`.
`=>` The stable form at room temperature is rhombic sulphur, which transforms to monoclinic sulphur when heated above `369 K`.