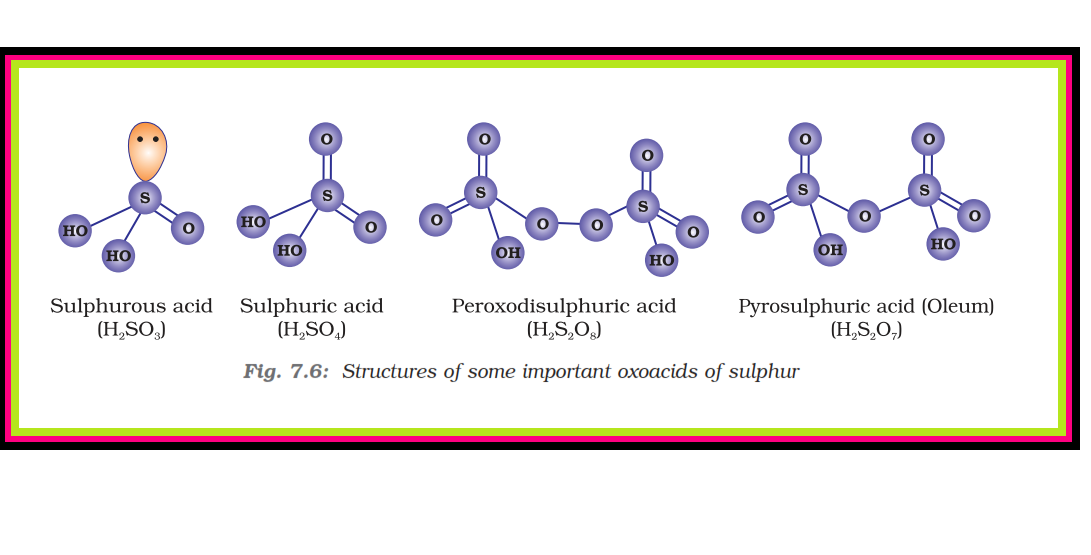
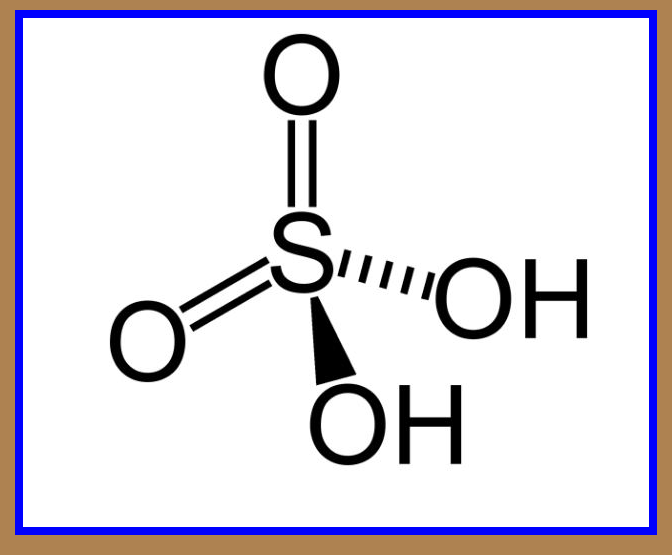
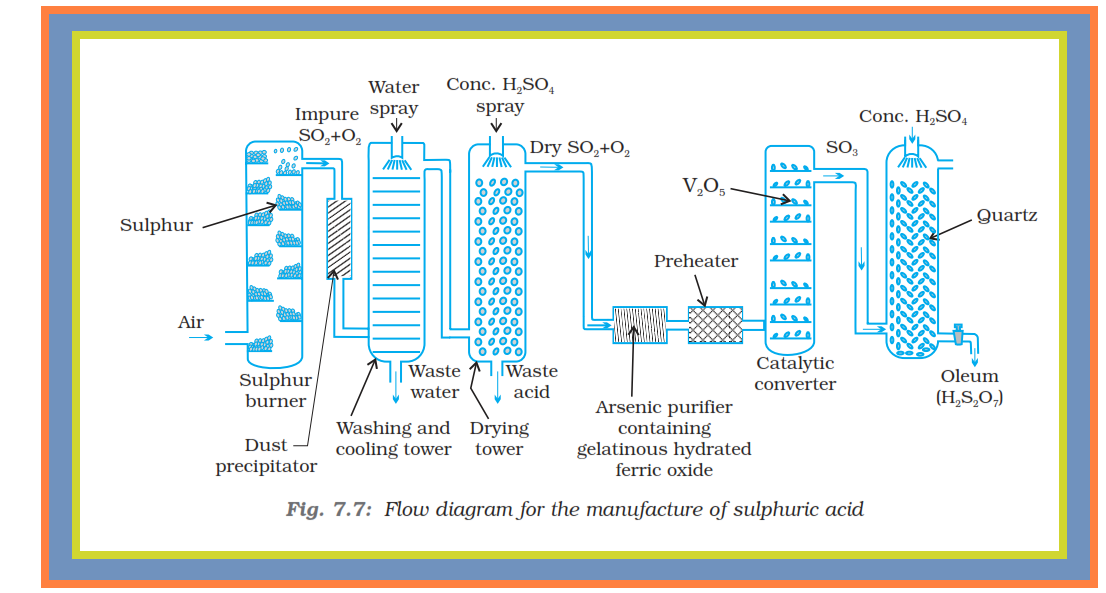
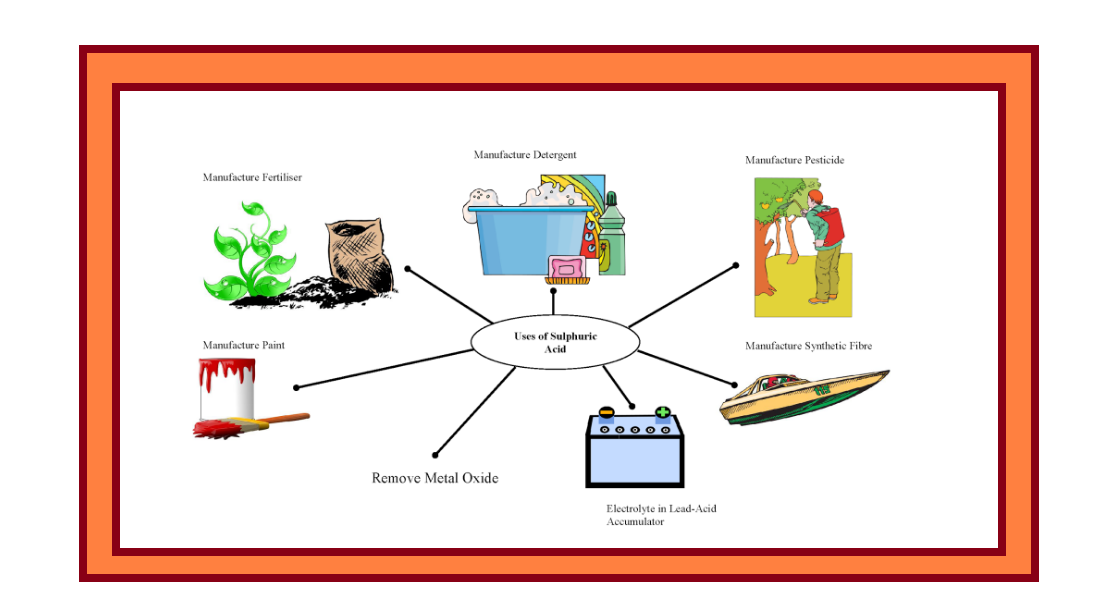
`=>` Sulphuric acid is a colourless, dense, oily liquid with a specific gravity of `1.84` at `298 K`.
`=>` The acid freezes at `283 K` and boils at `611 K`.
`=>` It dissolves in water with the evolution of a large quantity of heat. Hence, care must be taken while preparing sulphuric acid solution from concentrated sulphuric acid.
`=>` The concentrated acid must be added slowly into water with constant stirring.
`=>` The chemical reactions of sulphuric acid are as a result of the following characteristics : (a) low volatility (b) strong acidic character (c) strong affinity for water and (d) ability to act as an oxidising agent.
`=>` In aqueous solution, sulphuric acid ionises in two steps.
`color{red}(H_2SO_4 (aq) +H_2O (l) → H_3O^(+) (aq) +HSO^(-) (aq) ; K_(a_1) = text(very large) (k_(a_1) > 10))`
`color{red}(HSO_4^(-) (aq) +H_2O (l) → H_3 O^(+) (aq) +SO_4^(2-) (aq) ; K_(a_2) = 1.2xx10^(-2))`
The larger value of `color{red}(K_(a_1) (K_(a_1) >10))` means that `color{red}(H_2SO_4)` is largely dissociated into `color{red}(H^+)` and `color{red}(HSO_4^–)`. Greater the value of dissociation constant `color{red}(K_a)`, the stronger is the acid.
`=>` The acid forms two series of salts : normal sulphates (such as sodium sulphate and copper sulphate) and acid sulphates (e.g., sodium hydrogen sulphate).
`=>` Sulphuric acid, because of its low volatility can be used to manufacture more volatile acids from their corresponding salts.
`color{red}(2MX +H_2SO_4 → 2HX +M_2SO_4 ( X = F , Cl , NO_3) \ \ \ \ \ \ ( M = text(Metal )))`
`=>` Concentrated sulphuric acid is a strong dehydrating agent. Many wet gases can be dried by passing them through sulphuric acid,
provided the gases do not react with the acid. Sulphuric acid removes water from organic compounds; it is evident by its charring action on carbohydrates.
`color{red}(C_(12) H_(22) O_(11) overset(H_2SO_4)→ 12 C + 11 H_2O)`
`=>` Hot concentrated sulphuric acid is a moderately strong oxidising agent. In this respect, it is intermediate between phosphoric and nitric acids. Both metals and non-metals are oxidised by concentrated sulphuric acid, which is reduced to `color{red}(SO_2)`.
`color{red}(Cu +2H_2SO_4(conc.) → CuSO_4 +SO_2+2H_2O)`
`color{red}(3S +2H_2SO_4 (conc.) → 3SO_2+2H_2O)`
`color{red}(C +2H_2SO_4(conc.) → CO_2+2SO_2+2H_2O)`
`=>` Sulphuric acid is a colourless, dense, oily liquid with a specific gravity of `1.84` at `298 K`.
`=>` The acid freezes at `283 K` and boils at `611 K`.
`=>` It dissolves in water with the evolution of a large quantity of heat. Hence, care must be taken while preparing sulphuric acid solution from concentrated sulphuric acid.
`=>` The concentrated acid must be added slowly into water with constant stirring.
`=>` The chemical reactions of sulphuric acid are as a result of the following characteristics : (a) low volatility (b) strong acidic character (c) strong affinity for water and (d) ability to act as an oxidising agent.
`=>` In aqueous solution, sulphuric acid ionises in two steps.
`color{red}(H_2SO_4 (aq) +H_2O (l) → H_3O^(+) (aq) +HSO^(-) (aq) ; K_(a_1) = text(very large) (k_(a_1) > 10))`
`color{red}(HSO_4^(-) (aq) +H_2O (l) → H_3 O^(+) (aq) +SO_4^(2-) (aq) ; K_(a_2) = 1.2xx10^(-2))`
The larger value of `color{red}(K_(a_1) (K_(a_1) >10))` means that `color{red}(H_2SO_4)` is largely dissociated into `color{red}(H^+)` and `color{red}(HSO_4^–)`. Greater the value of dissociation constant `color{red}(K_a)`, the stronger is the acid.
`=>` The acid forms two series of salts : normal sulphates (such as sodium sulphate and copper sulphate) and acid sulphates (e.g., sodium hydrogen sulphate).
`=>` Sulphuric acid, because of its low volatility can be used to manufacture more volatile acids from their corresponding salts.
`color{red}(2MX +H_2SO_4 → 2HX +M_2SO_4 ( X = F , Cl , NO_3) \ \ \ \ \ \ ( M = text(Metal )))`
`=>` Concentrated sulphuric acid is a strong dehydrating agent. Many wet gases can be dried by passing them through sulphuric acid,
provided the gases do not react with the acid. Sulphuric acid removes water from organic compounds; it is evident by its charring action on carbohydrates.
`color{red}(C_(12) H_(22) O_(11) overset(H_2SO_4)→ 12 C + 11 H_2O)`
`=>` Hot concentrated sulphuric acid is a moderately strong oxidising agent. In this respect, it is intermediate between phosphoric and nitric acids. Both metals and non-metals are oxidised by concentrated sulphuric acid, which is reduced to `color{red}(SO_2)`.
`color{red}(Cu +2H_2SO_4(conc.) → CuSO_4 +SO_2+2H_2O)`
`color{red}(3S +2H_2SO_4 (conc.) → 3SO_2+2H_2O)`
`color{red}(C +2H_2SO_4(conc.) → CO_2+2SO_2+2H_2O)`