Oxidation states and trends in chemical reactivity :
`=>` All the halogens exhibit `-1` oxidation state.
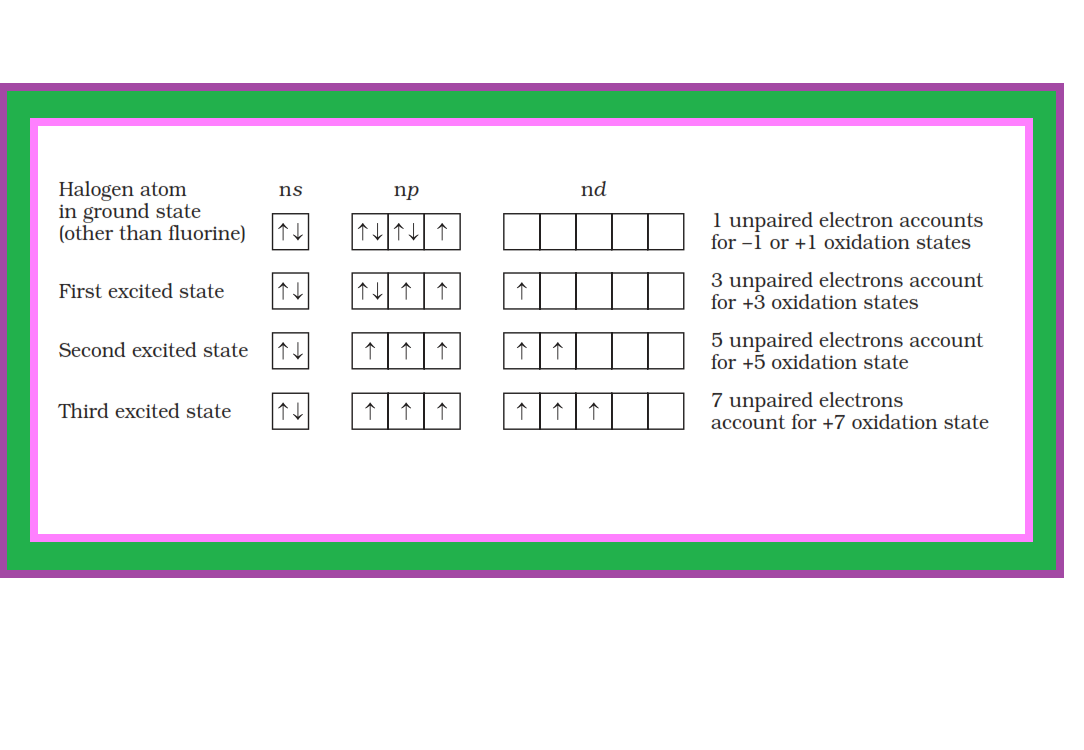
`=>` However, chlorine, bromine and iodine exhibit `+ 1`, `+ 3`, `+ 5` and `+ 7` oxidation states also as explained in Fig.
● The higher oxidation states of chlorine, bromine and iodine are realised mainly when the halogens are in combination with the small and highly electronegative fluorine and oxygen atoms. e.g., in interhalogens, oxides and oxoacids.
● The oxidation states of `+4` and `+6` occur in the oxides and oxoacids of chlorine and bromine.
● The fluorine atom has no `d`-orbitals in its valence shell and therefore cannot expand its octet.
● Being the most electronegative, it exhibits only `–1` oxidation state.
`=>` All the halogens are highly reactive. They react with metals and non-metals to form halides. The reactivity of the halogens decreases down the group.
`=>` The ready acceptance of an electron is the reason for the strong oxidising nature of halogens.
● `color{red}(F_2)` is the strongest oxidising halogen and it oxidises other halide ions in solution or even in the solid phase.
● In general, a halogen oxidises halide ions of higher atomic number.
`color{red}(F_2 + 2 X^(-) → 2F^(-) + X_2 ( X = Cl , Br text(or) I))`
`color{red}(Cl_2 +2X^(-) → 2Cl^(-) +X_2 ( X = Br text(or) I))`
`color{red}(Br_2 + 2I^(-) → 2Br^(-) +I_2)`
`=>` The decreasing oxidising ability of the halogens in aqueous solution down the group is evident from their standard electrode potentials (Table 7.8) which are dependent on the parameters indicated below:
`color{red}(1/2 X_2 (g) overset ( 1/2 Delta_(diss) H^(⊖) ) → X (g) overset (Delta(_eg)H^(⊖))→ X^(-) (s) overset (Delta _(hyd) H^(⊖)) →X^(-) (aq))`
`=>` The relative oxidising power of halogens can further be illustrated by their reactions with water.
● Fluorine oxidises water to oxygen whereas chlorine and bromine react with water to form corresponding hydrohalic and hypohalous acids.
● The reaction of iodine with water is nonspontaneous. In fact, `color{red}(I^–)` can be oxidised by oxygen in acidic medium; just the reverse of the reaction observed with fluorine.
`color{red}(2F_2 (g) +2H_2O(l) → 4H^(+) (aq) +4F^(-) (aq) +O_2 (g))`
`color{red}(X_2 (g) +H_2O (l) → HX (aq) +HOX (aq) \ \ \ \ ( text(where ) X = Cl text(or) Br))`
`color{red}(4I^(-) (aq) +4H^(+) (aq) +O_2 (g) → 2I_2 (s) +2H_2O(l))`
`=>` However, chlorine, bromine and iodine exhibit `+ 1`, `+ 3`, `+ 5` and `+ 7` oxidation states also as explained in Fig.
● The higher oxidation states of chlorine, bromine and iodine are realised mainly when the halogens are in combination with the small and highly electronegative fluorine and oxygen atoms. e.g., in interhalogens, oxides and oxoacids.
● The oxidation states of `+4` and `+6` occur in the oxides and oxoacids of chlorine and bromine.
● The fluorine atom has no `d`-orbitals in its valence shell and therefore cannot expand its octet.
● Being the most electronegative, it exhibits only `–1` oxidation state.
`=>` All the halogens are highly reactive. They react with metals and non-metals to form halides. The reactivity of the halogens decreases down the group.
`=>` The ready acceptance of an electron is the reason for the strong oxidising nature of halogens.
● `color{red}(F_2)` is the strongest oxidising halogen and it oxidises other halide ions in solution or even in the solid phase.
● In general, a halogen oxidises halide ions of higher atomic number.
`color{red}(F_2 + 2 X^(-) → 2F^(-) + X_2 ( X = Cl , Br text(or) I))`
`color{red}(Cl_2 +2X^(-) → 2Cl^(-) +X_2 ( X = Br text(or) I))`
`color{red}(Br_2 + 2I^(-) → 2Br^(-) +I_2)`
`=>` The decreasing oxidising ability of the halogens in aqueous solution down the group is evident from their standard electrode potentials (Table 7.8) which are dependent on the parameters indicated below:
`color{red}(1/2 X_2 (g) overset ( 1/2 Delta_(diss) H^(⊖) ) → X (g) overset (Delta(_eg)H^(⊖))→ X^(-) (s) overset (Delta _(hyd) H^(⊖)) →X^(-) (aq))`
`=>` The relative oxidising power of halogens can further be illustrated by their reactions with water.
● Fluorine oxidises water to oxygen whereas chlorine and bromine react with water to form corresponding hydrohalic and hypohalous acids.
● The reaction of iodine with water is nonspontaneous. In fact, `color{red}(I^–)` can be oxidised by oxygen in acidic medium; just the reverse of the reaction observed with fluorine.
`color{red}(2F_2 (g) +2H_2O(l) → 4H^(+) (aq) +4F^(-) (aq) +O_2 (g))`
`color{red}(X_2 (g) +H_2O (l) → HX (aq) +HOX (aq) \ \ \ \ ( text(where ) X = Cl text(or) Br))`
`color{red}(4I^(-) (aq) +4H^(+) (aq) +O_2 (g) → 2I_2 (s) +2H_2O(l))`