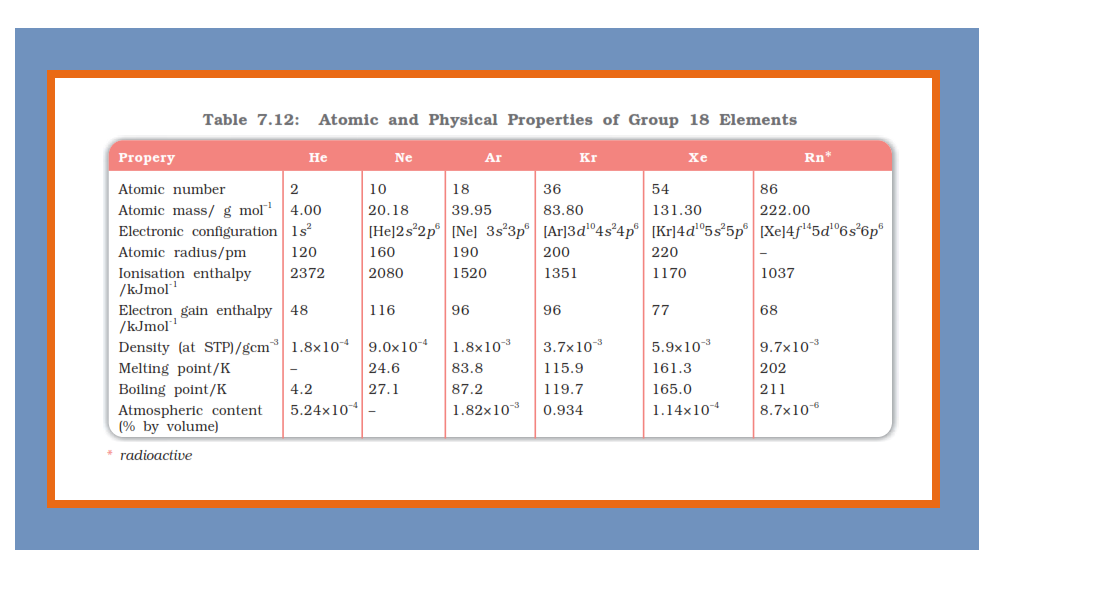
Group 18 Elements :
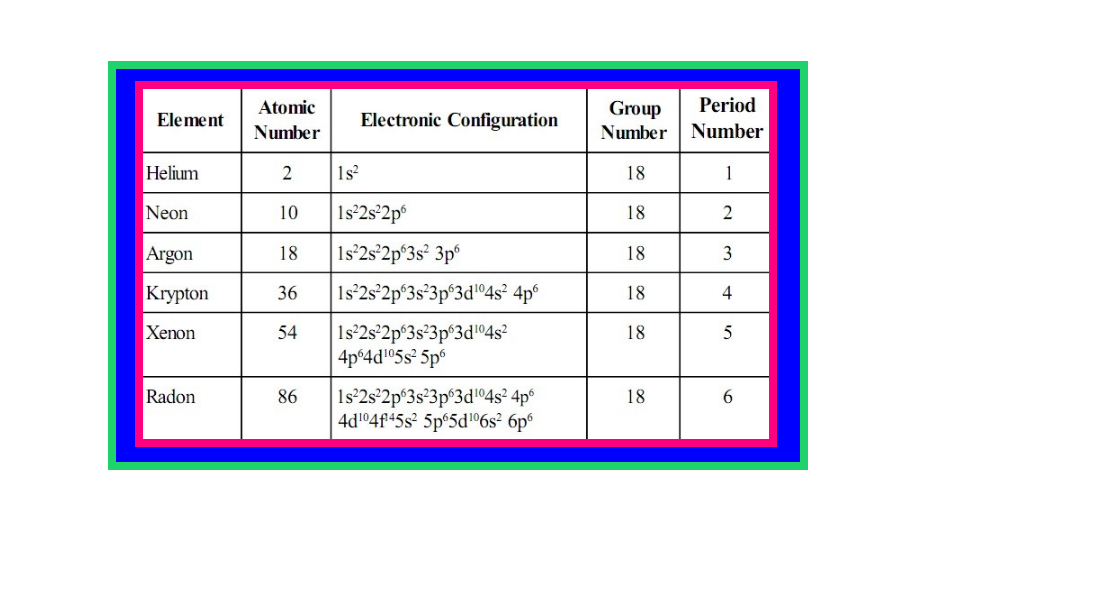
`=>` Group 18 consists of six elements : helium, neon, argon, krypton, xenon and radon.
`=>` All these are gases and chemically unreactive.
`=>` They form very few compounds. Because of this, they are termed noble gases.
`=>` All these are gases and chemically unreactive.
`=>` They form very few compounds. Because of this, they are termed noble gases.