Xenon-fluorine compounds :
`=>` Xenon forms three binary fluorides, `color{red}(XeF_2, XeF_4)` and `color{red}(XeF_6)` by the direct reaction of elements under appropriate experimental conditions.
`color{red}(undersettext{(xenon in excess)}(Xe(g)) +F_2 (g) overset(673 , text(1 bar))→ XeF_2 (s))`
`color{red}(undersettext{(1:5 ratio)} (Xe (g)) +2F_2 (g) overset(873K, text(7 bar))→ XeF_4 (s))`
`color{red}(undersettext{(1:20 ratio)} (Xe (g)) +3F_2 (g) oversettext(573 K, 60−70bar)→ XeF_6 (s))`
`=>` `color{red}(XeF_6)` can also be prepared by the interaction of `color{red}(XeF_4)` and `color{red}(O_2F_2)` at `143K`.
`color{red}(XeF_4 +O_2F_2 → XeF_6 +O_2)`
`=>` `color{red}(XeF_2`, `XeF_4)` and `color{red}(XeF_6)` are colourless crystalline solids and sublime readily at `298 K`.
`=>` They are powerful fluorinating agents.
`=>` They are readily hydrolysed even by traces of water. For example, `color{red}(XeF_2)` is hydrolysed to give `color{red}(Xe, HF)` and `color{red}(O_2)`.
`color{red}(2XeF_2 (s) + 2H_2O (l) → 2Xe (g)+4HF(aq) +O_2 (g))`
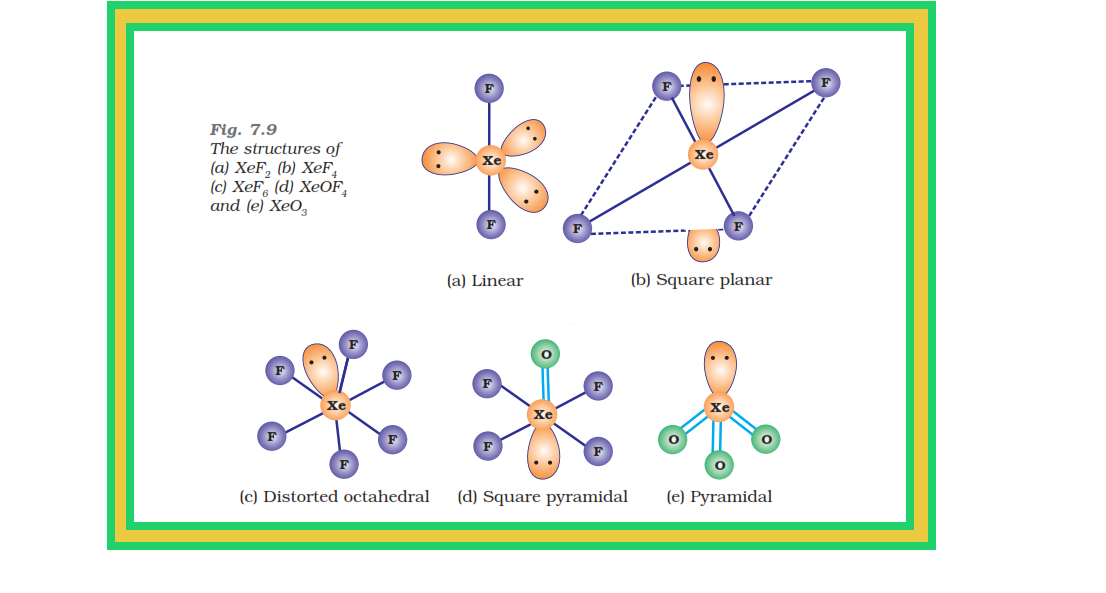
`=>` The structures of the three xenon fluorides can be deduced from VSEPR and these are shown in Fig. 7.9.
● `color{red}(XeF_2)` and `color{red}(XeF_4)` have linear and square planar structures respectively.
● `color{red}(XeF_6)` has seven electron pairs (6 bonding pairs and one lone pair) and would, thus, have a distorted octahedral structure as found experimentally in the gas phase.
`=>` Xenon fluorides react with fluoride ion acceptors to form cationic species and fluoride ion donors to form fluoroanions.
`color{red}(XeF_2+PF_5 → [XeF]^+ [PF_6]^(-) ; \ \ \ \ \ XeF_4 + SbF_5 → [XeF_3]^(+) [ SbF_6]^(-))`
`color{red}(XeF_6 + MF → M^(+) [XeF_7]^(-) \ \ (M = Na , K , Rb text(or) Cs))`
`color{red}(undersettext{(xenon in excess)}(Xe(g)) +F_2 (g) overset(673 , text(1 bar))→ XeF_2 (s))`
`color{red}(undersettext{(1:5 ratio)} (Xe (g)) +2F_2 (g) overset(873K, text(7 bar))→ XeF_4 (s))`
`color{red}(undersettext{(1:20 ratio)} (Xe (g)) +3F_2 (g) oversettext(573 K, 60−70bar)→ XeF_6 (s))`
`=>` `color{red}(XeF_6)` can also be prepared by the interaction of `color{red}(XeF_4)` and `color{red}(O_2F_2)` at `143K`.
`color{red}(XeF_4 +O_2F_2 → XeF_6 +O_2)`
`=>` `color{red}(XeF_2`, `XeF_4)` and `color{red}(XeF_6)` are colourless crystalline solids and sublime readily at `298 K`.
`=>` They are powerful fluorinating agents.
`=>` They are readily hydrolysed even by traces of water. For example, `color{red}(XeF_2)` is hydrolysed to give `color{red}(Xe, HF)` and `color{red}(O_2)`.
`color{red}(2XeF_2 (s) + 2H_2O (l) → 2Xe (g)+4HF(aq) +O_2 (g))`
`=>` The structures of the three xenon fluorides can be deduced from VSEPR and these are shown in Fig. 7.9.
● `color{red}(XeF_2)` and `color{red}(XeF_4)` have linear and square planar structures respectively.
● `color{red}(XeF_6)` has seven electron pairs (6 bonding pairs and one lone pair) and would, thus, have a distorted octahedral structure as found experimentally in the gas phase.
`=>` Xenon fluorides react with fluoride ion acceptors to form cationic species and fluoride ion donors to form fluoroanions.
`color{red}(XeF_2+PF_5 → [XeF]^+ [PF_6]^(-) ; \ \ \ \ \ XeF_4 + SbF_5 → [XeF_3]^(+) [ SbF_6]^(-))`
`color{red}(XeF_6 + MF → M^(+) [XeF_7]^(-) \ \ (M = Na , K , Rb text(or) Cs))`