Classification of Alcohols and Phenols :
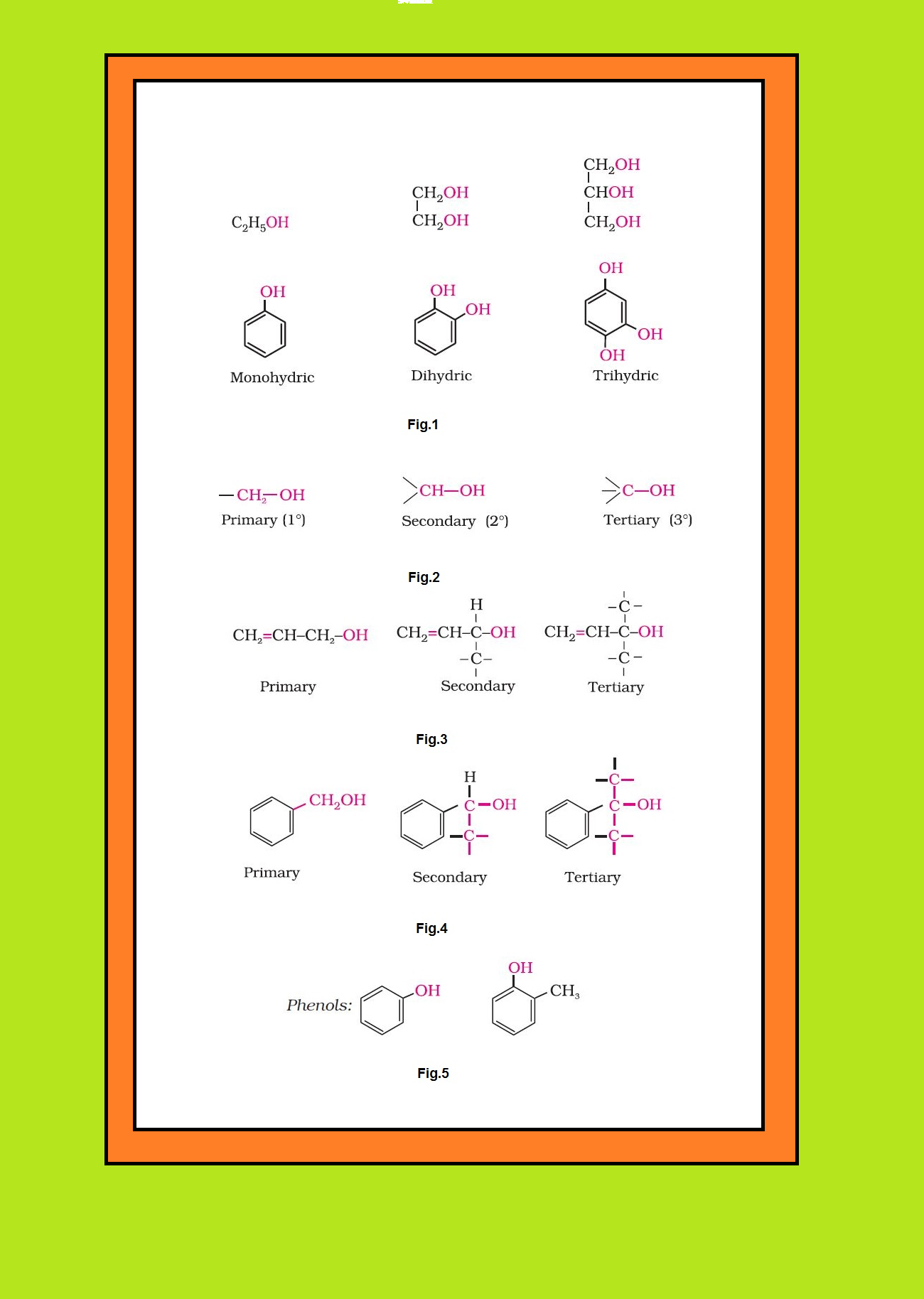
`text(Mono, Di, Tri or Polyhydric Compounds :)` Alcohols and phenols may be classified as mono–, di–, tri- or polyhydric compounds depending on whether they contain one, two, three or many hydroxyl groups respectively in their structures as given below : See fig.1.
`=>` Monohydric alcohols may be further classified according to the hybridisation of the carbon atom to which the hydroxyl group is attached.
(i) `color{green}("Compounds containing" C_(sp^3) - OH "bond ")` : In this class of alcohols, the `color{red}(–OH)` group is attached to an `color{red}(sp^3)` hybridised carbon atom of an alkyl group. They are further classified as follows :
● `color{green}("Primary, Secondary and Tertiary Alcohols" )` : In these three types of alcohols, the `color{red}(–OH)` group is attached to primary, secondary and tertiary carbon atom, respectively as shown in fig.2.
● `color{green}("Allylic Alcohols ")` : In these alcohols, the `color{red}(—OH)` group is attached to a `color{red}(sp^3)` hybridised carbon next to the carbon-carbon double bond, that is to an allylic carbon. For example : See fig.3.
● `color{green}("Benzylic Alcohols ")` : In these alcohols, the `color{red}(—OH)` group is attached to a `color{red}(sp^3)`—hybridised carbon atom next to an aromatic ring. For example : See fig.4.
(ii) `color{green}("Compounds containing" C_(sp^2)− OH "bond" )` : These alcohols contain `color{red}(—OH)` group bonded to a carbon-carbon double bond i.e., to a vinylic carbon or to an aryl carbon. These alcohols are also known as vinylic alcohols.
Vinylic alcohol : `color{red}(CH_2 = CH – OH)`
Phenols : See fig.5.
`=>` Monohydric alcohols may be further classified according to the hybridisation of the carbon atom to which the hydroxyl group is attached.
(i) `color{green}("Compounds containing" C_(sp^3) - OH "bond ")` : In this class of alcohols, the `color{red}(–OH)` group is attached to an `color{red}(sp^3)` hybridised carbon atom of an alkyl group. They are further classified as follows :
● `color{green}("Primary, Secondary and Tertiary Alcohols" )` : In these three types of alcohols, the `color{red}(–OH)` group is attached to primary, secondary and tertiary carbon atom, respectively as shown in fig.2.
● `color{green}("Allylic Alcohols ")` : In these alcohols, the `color{red}(—OH)` group is attached to a `color{red}(sp^3)` hybridised carbon next to the carbon-carbon double bond, that is to an allylic carbon. For example : See fig.3.
● `color{green}("Benzylic Alcohols ")` : In these alcohols, the `color{red}(—OH)` group is attached to a `color{red}(sp^3)`—hybridised carbon atom next to an aromatic ring. For example : See fig.4.
(ii) `color{green}("Compounds containing" C_(sp^2)− OH "bond" )` : These alcohols contain `color{red}(—OH)` group bonded to a carbon-carbon double bond i.e., to a vinylic carbon or to an aryl carbon. These alcohols are also known as vinylic alcohols.
Vinylic alcohol : `color{red}(CH_2 = CH – OH)`
Phenols : See fig.5.