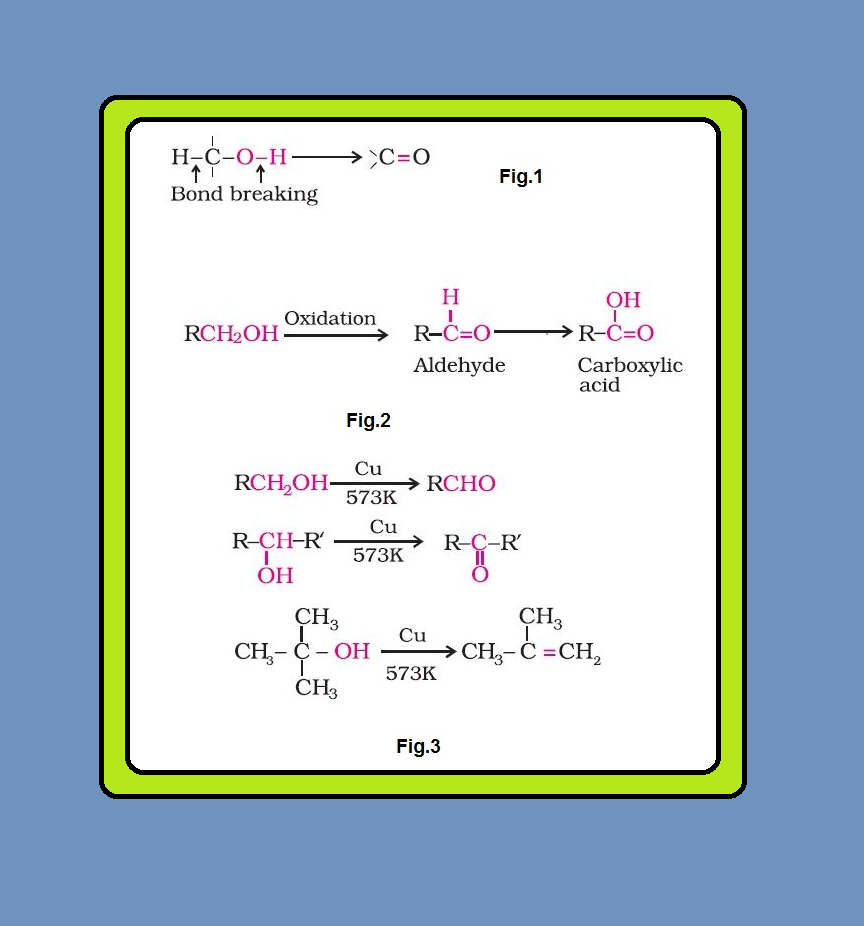
Dehydration :
`=>` Alcohols undergo dehydration (removal of a molecule of water) to form alkenes on treating with a protic acid e.g., concentrated `color{red}(H_2SO_4)` or `color{red}(H_3PO_4)`, or catalysts such as anhydrous zinc chloride or alumina (See fig.1).
Ethanol undergoes dehydration by heating it with concentrated `color{red}(H_2SO_4)` at `443 K`.
`color{red}(C_2H_5 OH underset(443 K) overset(H_2SO_4)→ CH_2 = CH_2+H_2O)`
Secondary and tertiary alcohols are dehydrated under milder conditions. For example
`color{red}(CH_3 overset(OH)CHCH_3 underset(440K) overset(85 % H_3PO_4)→CH_3- CH= CH_2+H_2O)`
`color{red}(CH_3 - underset ( underset (CH_3) (|)) overset ( overset(CH_3) (|))C - OH underset (358K) overset(20 % H_3PO_4)→ CH_3 - overset(overset(CH_3)(||))C - CH_3+H_2O)`
Thus, the relative ease of dehydration of alcohols follows the following order :
Tertiary > Secondary > Primary
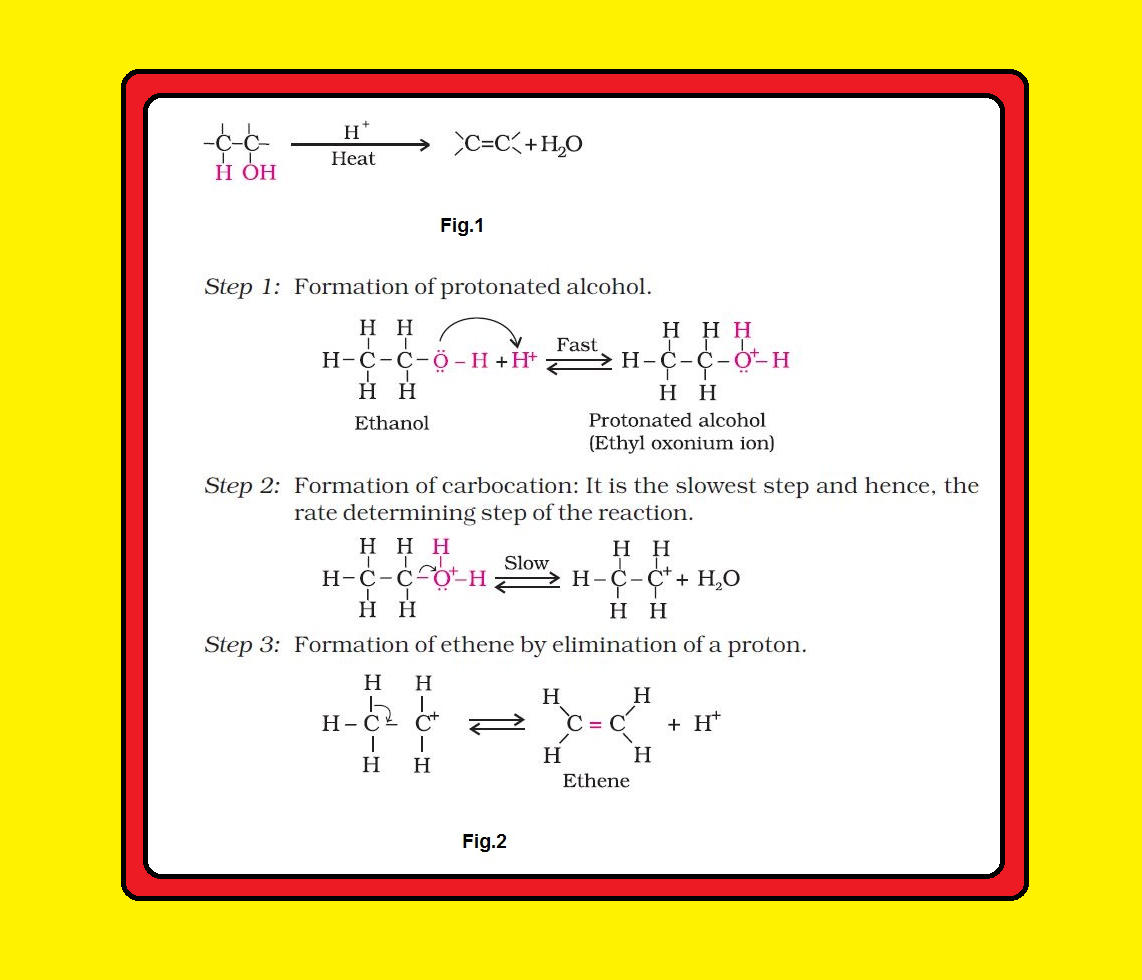
The mechanism of dehydration of ethanol involves the following steps:
Mechanism : See fig.2.
`color{red}("Note ")` ● The acid used in step 1 is released in step 3.
● To drive the equilibrium to the right, ethene is removed as it is formed.
Ethanol undergoes dehydration by heating it with concentrated `color{red}(H_2SO_4)` at `443 K`.
`color{red}(C_2H_5 OH underset(443 K) overset(H_2SO_4)→ CH_2 = CH_2+H_2O)`
Secondary and tertiary alcohols are dehydrated under milder conditions. For example
`color{red}(CH_3 overset(OH)CHCH_3 underset(440K) overset(85 % H_3PO_4)→CH_3- CH= CH_2+H_2O)`
`color{red}(CH_3 - underset ( underset (CH_3) (|)) overset ( overset(CH_3) (|))C - OH underset (358K) overset(20 % H_3PO_4)→ CH_3 - overset(overset(CH_3)(||))C - CH_3+H_2O)`
Thus, the relative ease of dehydration of alcohols follows the following order :
Tertiary > Secondary > Primary
The mechanism of dehydration of ethanol involves the following steps:
Mechanism : See fig.2.
`color{red}("Note ")` ● The acid used in step 1 is released in step 3.
● To drive the equilibrium to the right, ethene is removed as it is formed.