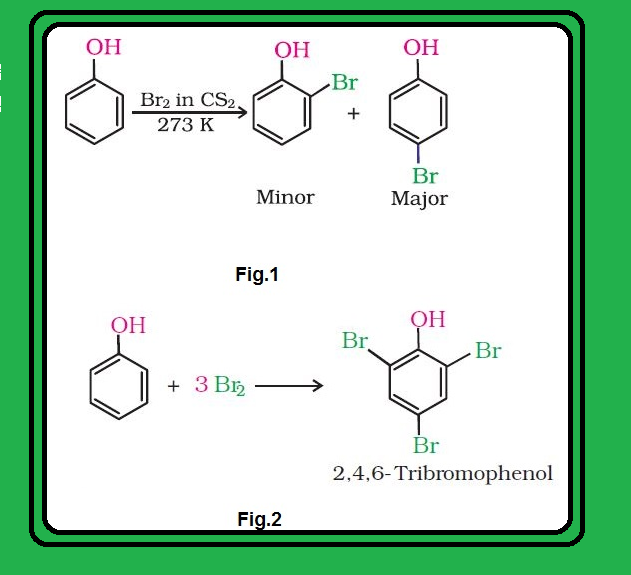
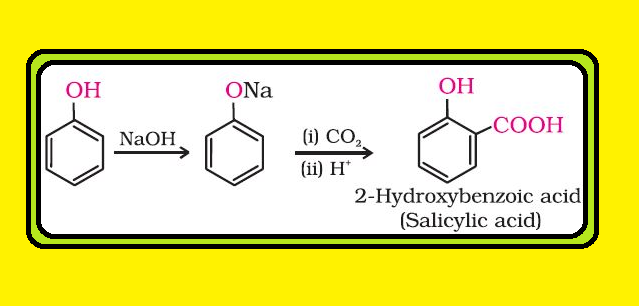
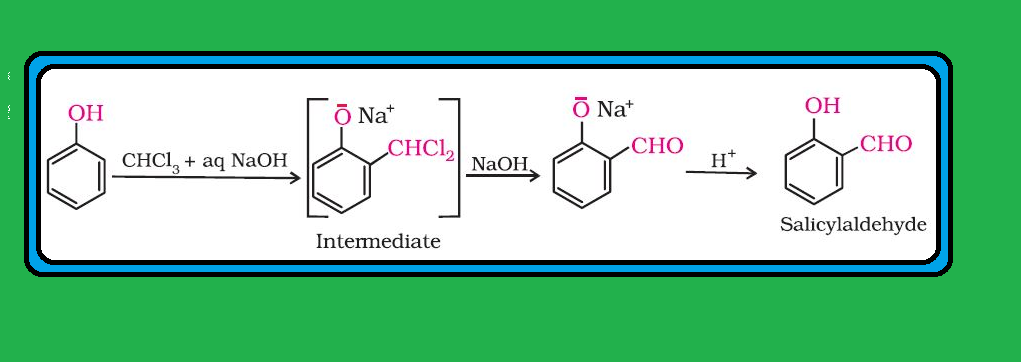
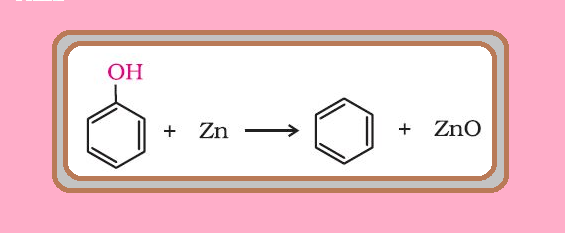
Nitration :
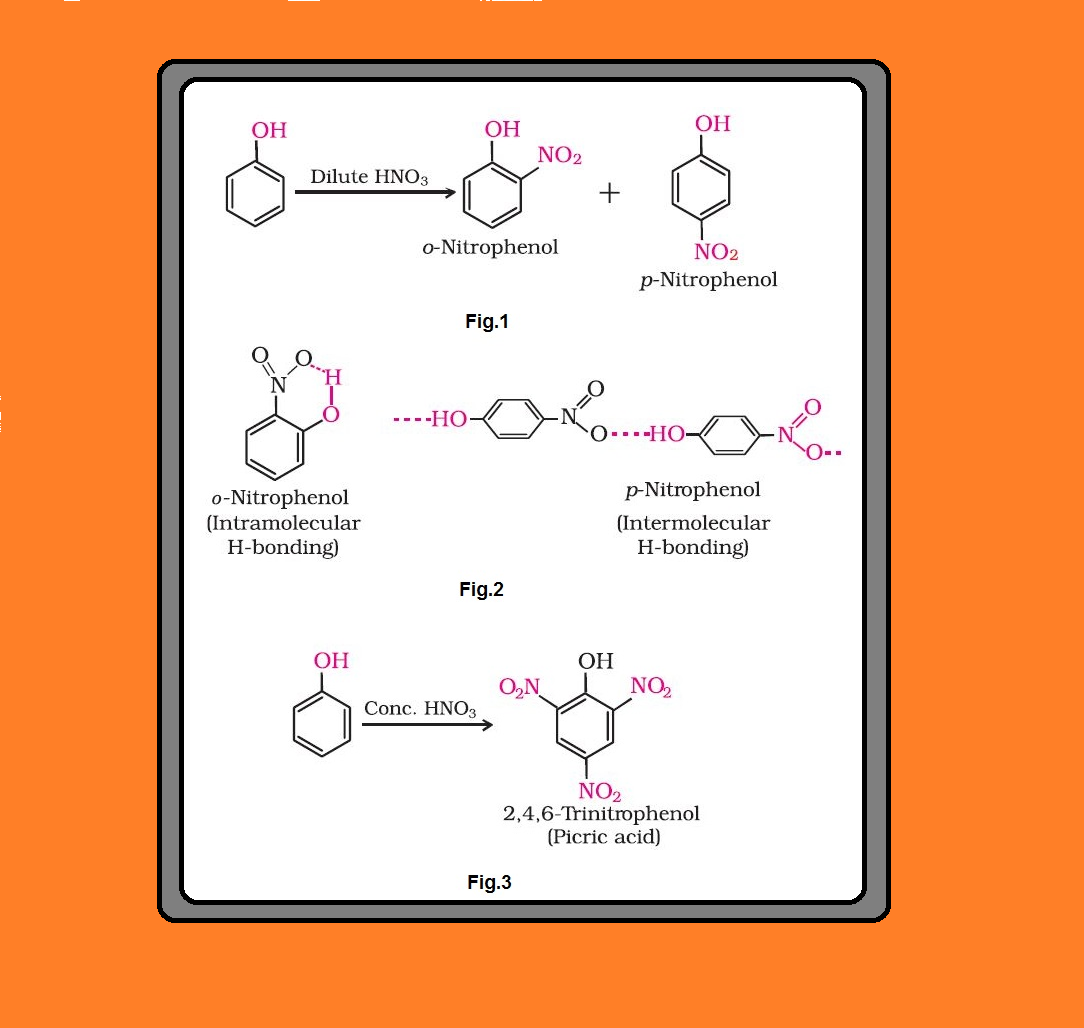
`=>` With dilute nitric acid at low temperature (`298 K`), phenol yields a mixture of ortho and para nitrophenols. See fig.1.
● The ortho and para isomers can be separated by steam distillation.
● o-Nitrophenol is steam volatile due to intramolecular hydrogen bonding while p-nitrophenol is less volatile due to intermolecular hydrogen bonding which causes the association of molecules. See fig.2.
● With concentrated nitric acid, phenol is converted to 2,4,6-trinitrophenol. The product is commonly known as picric acid. The yield of the reaction product is poor. See fig.3.
● 2, 4, 6 - Trinitrophenol is a strong acid due to the presence of three electron withdrawing `color{red}(–NO_2)` groups which facilitate the release of hydrogen ion.
● Picric acid is prepared by treating phenol first with concentrated sulphuric acid which converts it to phenol-2,4-disulphonic acid, and then with concentrated nitric acid to get 2,4,6-trinitrophenol.
● The ortho and para isomers can be separated by steam distillation.
● o-Nitrophenol is steam volatile due to intramolecular hydrogen bonding while p-nitrophenol is less volatile due to intermolecular hydrogen bonding which causes the association of molecules. See fig.2.
● With concentrated nitric acid, phenol is converted to 2,4,6-trinitrophenol. The product is commonly known as picric acid. The yield of the reaction product is poor. See fig.3.
● 2, 4, 6 - Trinitrophenol is a strong acid due to the presence of three electron withdrawing `color{red}(–NO_2)` groups which facilitate the release of hydrogen ion.
● Picric acid is prepared by treating phenol first with concentrated sulphuric acid which converts it to phenol-2,4-disulphonic acid, and then with concentrated nitric acid to get 2,4,6-trinitrophenol.