By dehydration of alcohols :
`=>` Alcohols undergo dehydration in the presence of protic acids `color{red}(H_2SO_4, H_3PO_4)`.
`=>` The formation of the reaction product, alkene or ether depends on the reaction conditions.
`=>` Example : Ethanol is dehydrated to ethene in the presence of sulphuric acid at `443 K`. At `413 K`, ethoxyethane is the main product.
`color{red}(CH_3CH_2OH underset(443K) overset(H_2SO_4)→ CH_2 = CH_2)`
`color{red}(CH_3CH_2OH underset(413K) overset(H_2SO_4)→ C_2H_5OC_2H_5)`
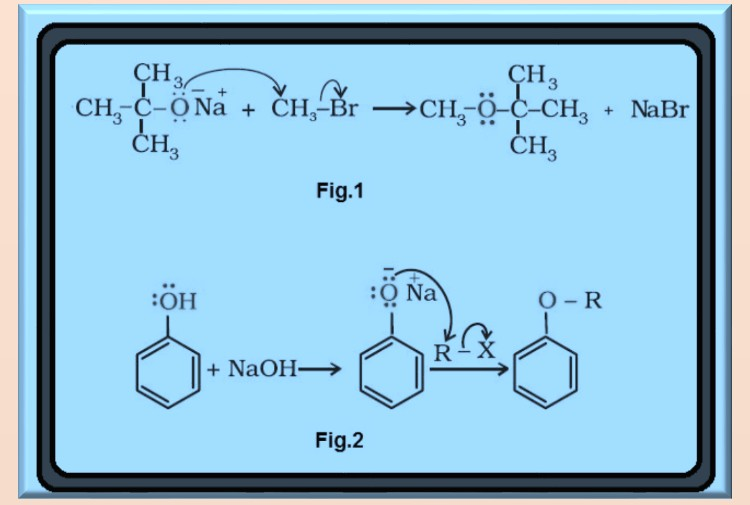
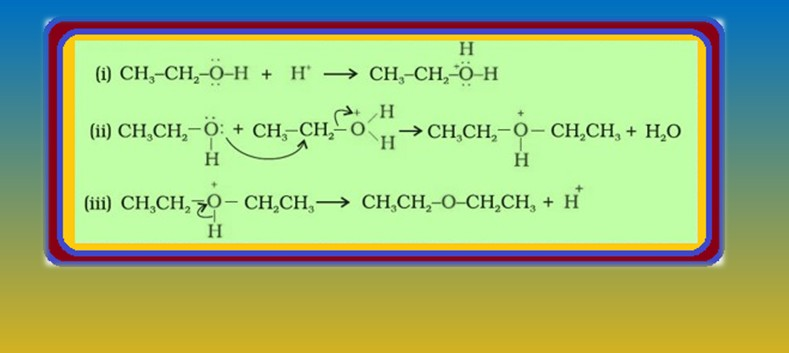
`=>` The formation of ether is a nucleophilic bimolecular reaction `color{red}(S_N 2)` involving the attack of alcohol molecule on a protonated alcohol, as shown in fig.
`=>` Acidic dehydration of alcohols, to give an alkene is also associated with substitution reaction to give an ether.
`=>` This method is suitable for the preparation of ethers having primary alkyl groups only.
● The alkyl group should be unhindered and the temperature be kept low. Otherwise the reaction favours the formation of alkene.
`=>` The reaction follows `color{red}(S_N 1)` pathway when the alcohol is secondary or tertiary.
● However, the dehydration of secondary and tertiary alcohols to give corresponding ethers is unsuccessful as elimination competes over substitution and as a consequence, alkenes are easily formed.
`=>` The formation of the reaction product, alkene or ether depends on the reaction conditions.
`=>` Example : Ethanol is dehydrated to ethene in the presence of sulphuric acid at `443 K`. At `413 K`, ethoxyethane is the main product.
`color{red}(CH_3CH_2OH underset(443K) overset(H_2SO_4)→ CH_2 = CH_2)`
`color{red}(CH_3CH_2OH underset(413K) overset(H_2SO_4)→ C_2H_5OC_2H_5)`
`=>` The formation of ether is a nucleophilic bimolecular reaction `color{red}(S_N 2)` involving the attack of alcohol molecule on a protonated alcohol, as shown in fig.
`=>` Acidic dehydration of alcohols, to give an alkene is also associated with substitution reaction to give an ether.
`=>` This method is suitable for the preparation of ethers having primary alkyl groups only.
● The alkyl group should be unhindered and the temperature be kept low. Otherwise the reaction favours the formation of alkene.
`=>` The reaction follows `color{red}(S_N 1)` pathway when the alcohol is secondary or tertiary.
● However, the dehydration of secondary and tertiary alcohols to give corresponding ethers is unsuccessful as elimination competes over substitution and as a consequence, alkenes are easily formed.