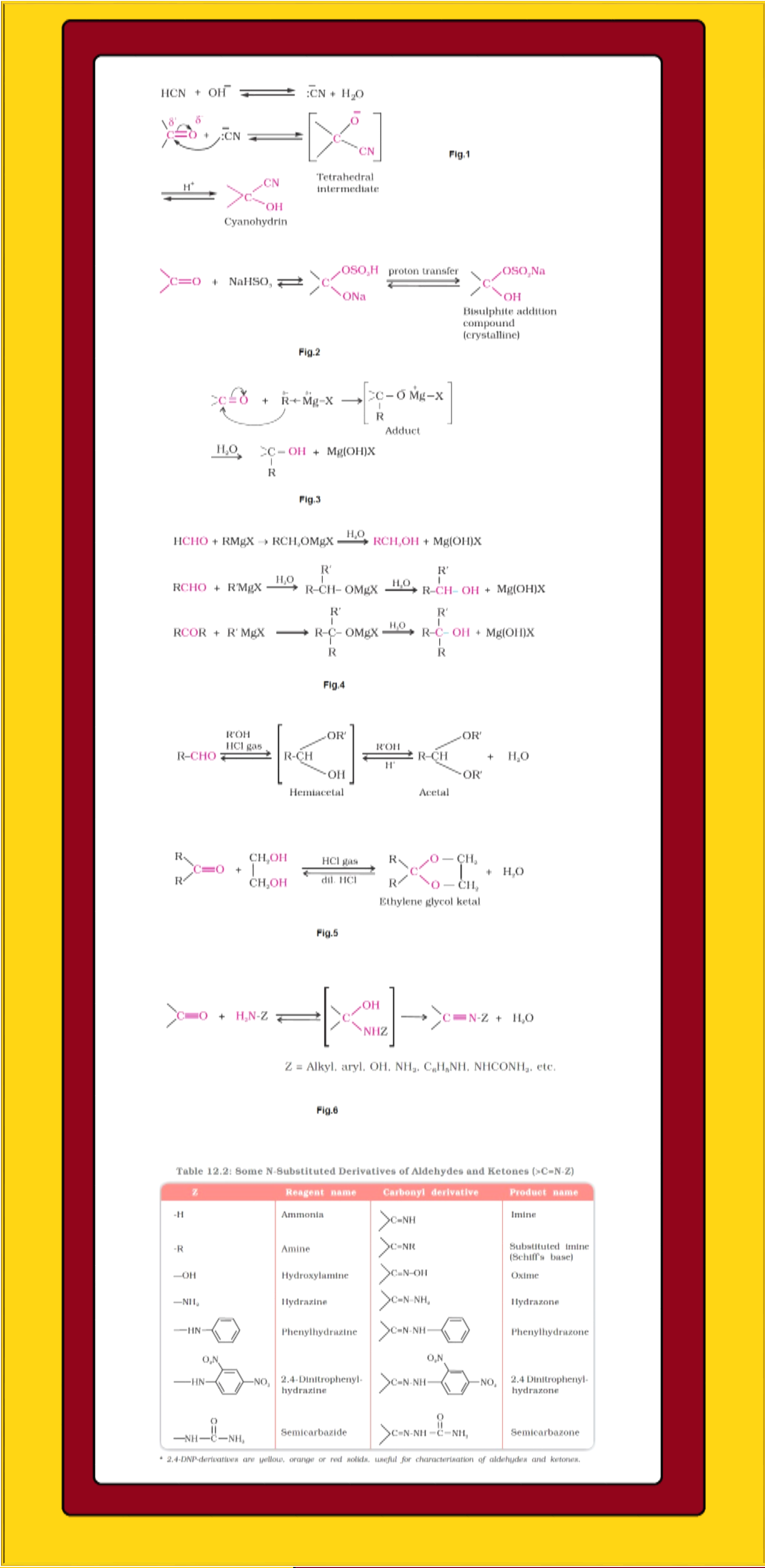
Mechanism of nucleophilic addition reactions :
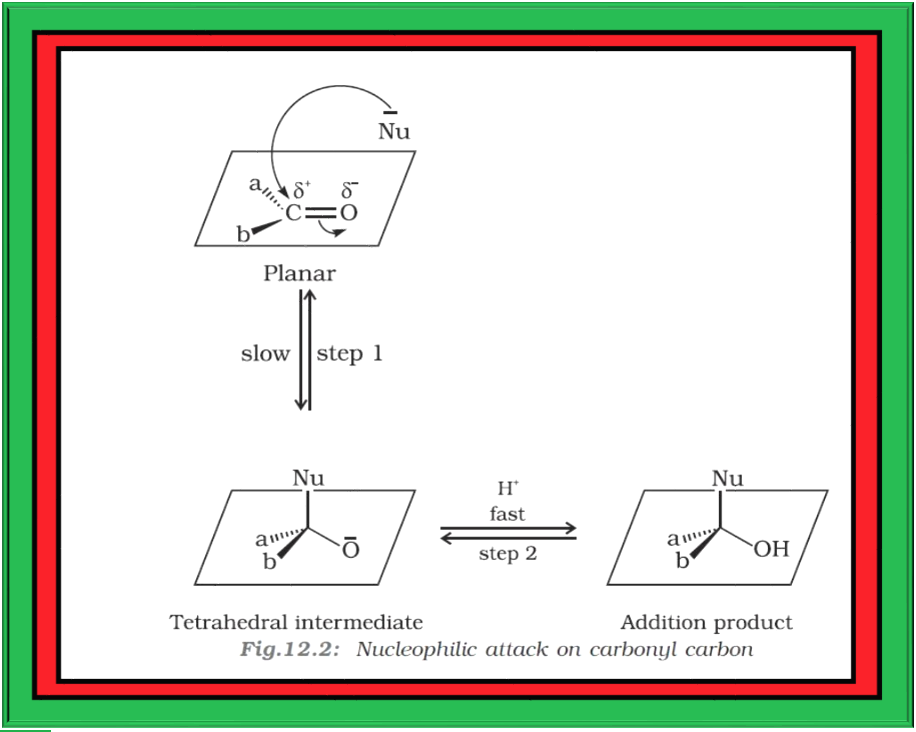
`=>` A nucleophile attacks the electrophilic carbon atom of the polar carbonyl group from a direction approximately perpendicular to the plane of `color{red}(sp^2)` hybridised orbitals of carbonyl carbon (Fig. 12.2).
`=>` The hybridisation of carbon changes from `color{red}(sp^2)` to `color{red}(sp^3)` in this process, and a tetrahedral alkoxide intermediate is produced.
`=>` This intermediate captures a proton from the reaction medium to give the electrically neutral product.
`=>` The net result is addition of `color{red}(Nu^–)` and `color{red}(H^+)` across the carbon oxygen double bond as shown in Fig. 12.2.
`=>` The hybridisation of carbon changes from `color{red}(sp^2)` to `color{red}(sp^3)` in this process, and a tetrahedral alkoxide intermediate is produced.
`=>` This intermediate captures a proton from the reaction medium to give the electrically neutral product.
`=>` The net result is addition of `color{red}(Nu^–)` and `color{red}(H^+)` across the carbon oxygen double bond as shown in Fig. 12.2.