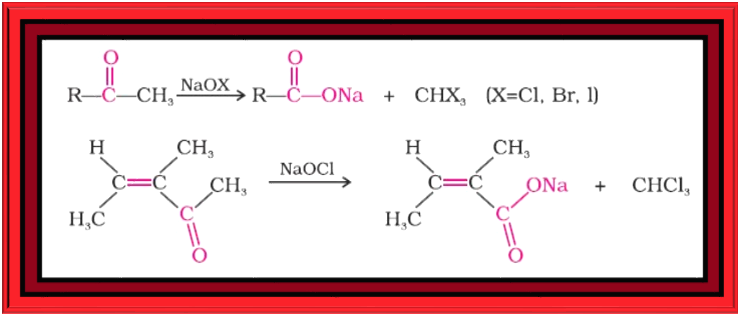
Reduction :
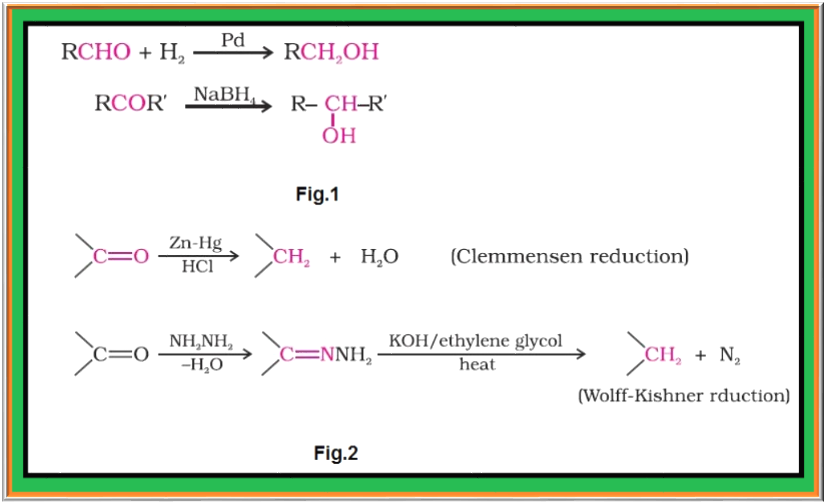
(i) `color{green}(text(Reduction to Alcohols ))` : Aldehydes and ketones are reduced to primary and secondary alcohols respectively by sodium borohydride `color{red}((NaBH_4))` or lithium aluminium hydride `color{red}((LiAlH_4))` as well as by catalytic hydrogenation. See fig.1.
(ii) `color{green}(text(Reduction to Hydrocarbons ))` : The carbonyl group of aldehydes and ketones is reduced to `color{red}(CH_2)` group on treatment with zinc amalgam and concentrated hydrochloric acid [Clemmensen reduction] or with hydrazine followed by heating with sodium or potassium hydroxide in high boiling solvent such as ethylene glycol (`color{green}(text(Wolff-Kishner reduction))`). See fig.2.
(ii) `color{green}(text(Reduction to Hydrocarbons ))` : The carbonyl group of aldehydes and ketones is reduced to `color{red}(CH_2)` group on treatment with zinc amalgam and concentrated hydrochloric acid [Clemmensen reduction] or with hydrazine followed by heating with sodium or potassium hydroxide in high boiling solvent such as ethylene glycol (`color{green}(text(Wolff-Kishner reduction))`). See fig.2.