Nomenclature :
`=>` Since carboxylic acids are amongst the earliest organic compounds to be isolated from nature, a large number of them are known by their common names.
`color{green}(text(Common Names :))` The common names end with the suffix `color{red}(–ic)` acid and have been derived from Latin or Greek names of their natural sources.
● `color{green}(text(For example :))` Formic acid `color{red}((HCOOH))` was first obtained from red ants `color{green}(("Latin: formica means ant"))`, acetic acid `color{red}((CH_3COOH))` from vinegar `color{green}(("Latin: acetum, means vinegar"))`, butyric acid `color{red}((CH_3CH_2CH_2COOH))` from rancid butter `color{green}(("Latin: butyrum, means butter"))`.
`color{green}(text(IUPAC Name :))` In the IUPAC system, aliphatic carboxylic acids are named by replacing the ending `color{red}(–e)` in the name of the corresponding alkane with `color{red}(– oic)` acid.
● In numbering the carbon chain, the carboxylic carbon is numbered one.
● For naming compounds containing more than one carboxyl group, the ending `color{red}(–e)` of the alkane is retained.
● The number of carboxyl groups are indicated by adding the multiplicative prefix, di, tri, etc. to the term oic.
● The position of `color{red}(–COOH)` groups are indicated by the arabic numeral before the multiplicative prefix.
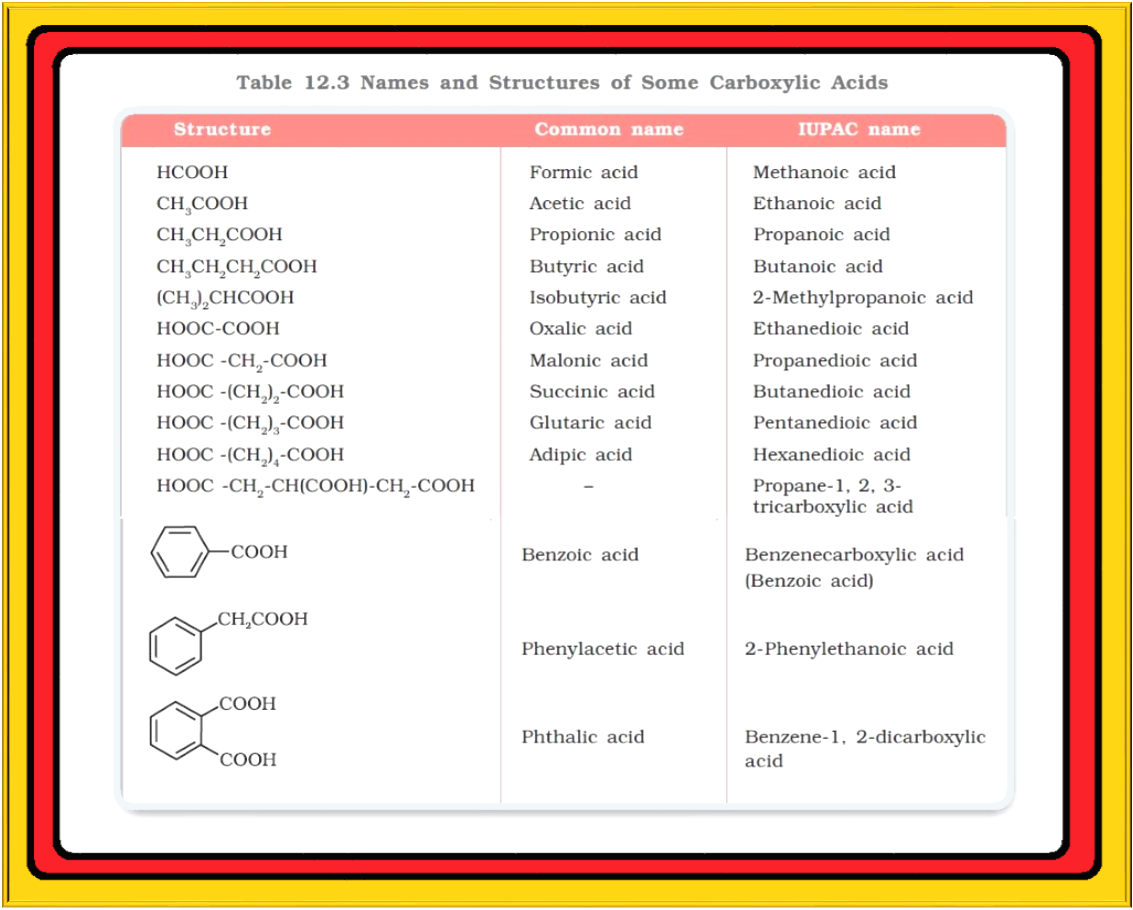
● Some of the carboxylic acids along with their common and IUPAC names are listed in Table 12.3.
`color{green}(text(Common Names :))` The common names end with the suffix `color{red}(–ic)` acid and have been derived from Latin or Greek names of their natural sources.
● `color{green}(text(For example :))` Formic acid `color{red}((HCOOH))` was first obtained from red ants `color{green}(("Latin: formica means ant"))`, acetic acid `color{red}((CH_3COOH))` from vinegar `color{green}(("Latin: acetum, means vinegar"))`, butyric acid `color{red}((CH_3CH_2CH_2COOH))` from rancid butter `color{green}(("Latin: butyrum, means butter"))`.
`color{green}(text(IUPAC Name :))` In the IUPAC system, aliphatic carboxylic acids are named by replacing the ending `color{red}(–e)` in the name of the corresponding alkane with `color{red}(– oic)` acid.
● In numbering the carbon chain, the carboxylic carbon is numbered one.
● For naming compounds containing more than one carboxyl group, the ending `color{red}(–e)` of the alkane is retained.
● The number of carboxyl groups are indicated by adding the multiplicative prefix, di, tri, etc. to the term oic.
● The position of `color{red}(–COOH)` groups are indicated by the arabic numeral before the multiplicative prefix.
● Some of the carboxylic acids along with their common and IUPAC names are listed in Table 12.3.
