Crystal field splitting in octahedral coordination entities :
`=>` In an octahedral coordination entity with six ligands surrounding the metal atom/ion, there will be repulsion between the electrons in metal `d`-orbitals and the electrons (or negative charges) of the ligands.
● Such a repulsion is more when the metal `d`-orbital is directed towards the ligand than when it is away from the ligand.
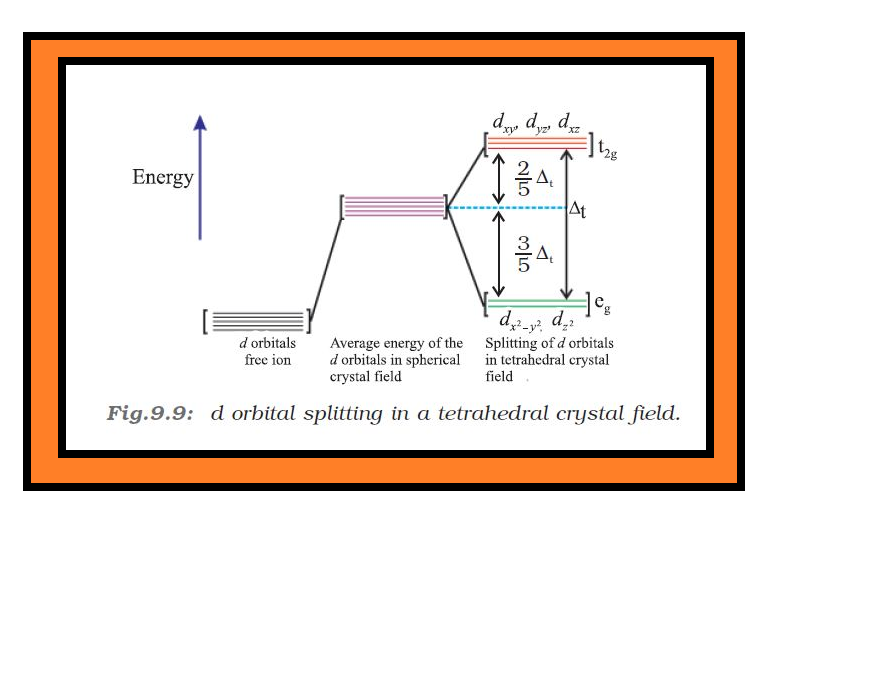
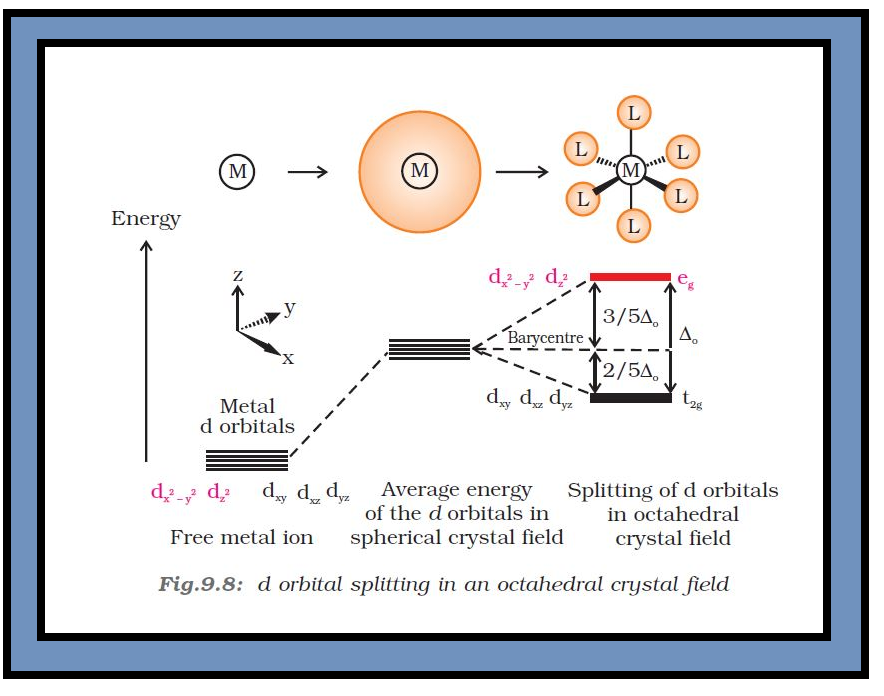
`=>` Therefore, the `color{red}(d_(x^2 − y^2))` and `color{red}(d_(z^2))` orbitals which point towards the axes along the direction of the ligand will experience more repulsion and will be raised in energy; and the `color{red}(d_(xy), d_(yz))` and `color{red}(d_(xz))` orbitals which are directed between the axes will be lowered in energy relative to the average energy in the spherical crystal field.
`=>` Thus, the degeneracy of the `d` orbitals has been removed due to ligand electron-metal electron repulsions in the octahedral complex to yield three orbitals of lower energy, `color{red}(t_(2g))` set and two orbitals of higher energy, `color{red}(e_g)` set.
`=>` This splitting of the degenerate levels due to the presence of ligands in a definite geometry is termed as crystal field splitting and the energy separation is denoted by `color{red}(Δ_o)` (the subscript `o` is for octahedral) (Fig.9.8).
`=>` Thus, the energy of the two `color{red}(e_g)` orbitals will increase by `color{red}((3/5) Δ_o)` and that of the three `color{red}(t_(2g))` will decrease by `color{red}((2/5)Δ_o)`.
`=>` The crystal field splitting, `color{red}(Δ_o)`, depends upon the field produced by the ligand and charge on the metal ion.
`=>` Some ligands are able to produce strong fields in which case, the splitting will be large whereas others produce weak fields and consequently result in small splitting of `d`-orbitals.
● In general, ligands can be arranged in a series in the order of increasing field strength as given below :
`color{red}(I^– < Br^– < SCN^– < Cl^– < S2^– < F^– < OH^– < C_2O_4^(2–) < H_2O < NCS^– < edta^(4–) < NH_3 < en < CN^(–) < CO)`
● Such a series is termed as `color{green}("spectrochemical series")`.
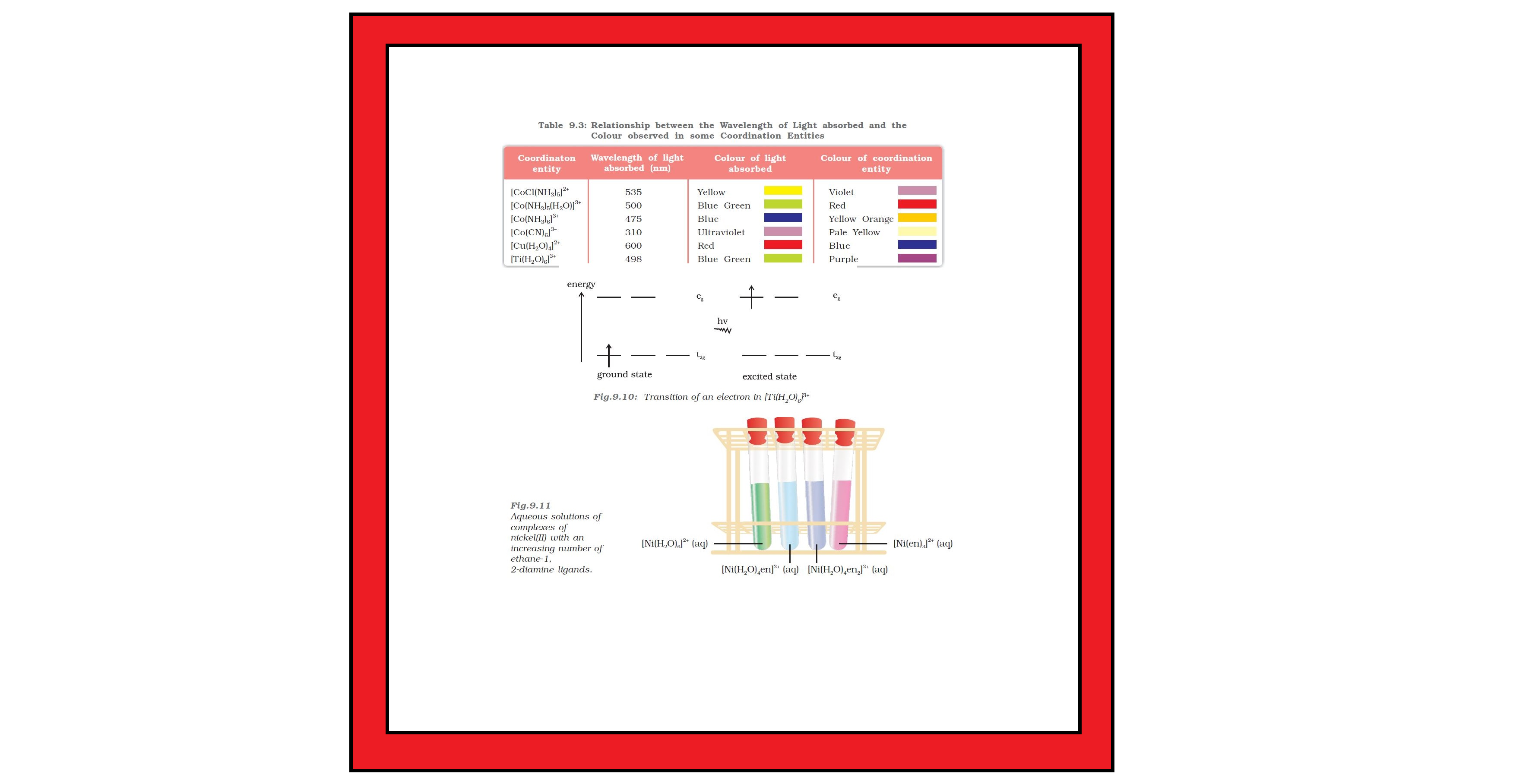
● It is an experimentally determined series based on the absorption of light by complexes with different ligands.
`=>` Let us assign electrons in the `color{red}(d)`-orbitals of metal ion in octahedral coordination entities.
● Obviously, the single `color{red}(d)` electron occupies one of the lower energy `color{red}(t_(2g))` orbitals.
● In `color{red}(d^2)` and `color{red}(d^3)` coordination entities, the `color{red}(d)` electrons occupy the `color{red}(t_(2g))` orbitals singly in accordance with the Hund’s rule.
● For `color{red}(d^4)` ions, two possible patterns of electron distribution arise :
(i) the fourth electron could either enter the `color{red}(t_(2g))` level and pair with an existing electron, or
(ii) it could avoid paying the price of the pairing energy by occupying the `color{red}(e_g)` level.
● Which of these possibilities occurs, depends on the relative magnitude of the crystal field splitting, `color{red}(Δ_o)` and the pairing energy, `P` (`P` represents the energy required for electron pairing in a single orbital).
● The two options are :
(i) If `color{red}(Δ_o < P)`, the fourth electron enters one of the `color{red}(e_g)` orbitals giving the configuration `color{red}(t_(2g)^3 e_(g)^1)`. Ligands for which `color{red}(Δ_o < P)` are known as weak field ligands and form high spin complexes.
(ii) If `color{red}(Δ_o > P)`, it becomes more energetically favourable for the fourth electron to occupy a `color{red}(t_(2g))` orbital with configuration `color{red}(t_(2g)^4 e_(g)^0)`. Ligand which produce this effect are known as strong field ligands and form low spin complexes.
`color{red}("Note ")` : Calculations show that `color{red}(d^4)` to `color{red}(d^7)` coordination entities are more stable for strong field as compared to weak field cases.
● Such a repulsion is more when the metal `d`-orbital is directed towards the ligand than when it is away from the ligand.
`=>` Therefore, the `color{red}(d_(x^2 − y^2))` and `color{red}(d_(z^2))` orbitals which point towards the axes along the direction of the ligand will experience more repulsion and will be raised in energy; and the `color{red}(d_(xy), d_(yz))` and `color{red}(d_(xz))` orbitals which are directed between the axes will be lowered in energy relative to the average energy in the spherical crystal field.
`=>` Thus, the degeneracy of the `d` orbitals has been removed due to ligand electron-metal electron repulsions in the octahedral complex to yield three orbitals of lower energy, `color{red}(t_(2g))` set and two orbitals of higher energy, `color{red}(e_g)` set.
`=>` This splitting of the degenerate levels due to the presence of ligands in a definite geometry is termed as crystal field splitting and the energy separation is denoted by `color{red}(Δ_o)` (the subscript `o` is for octahedral) (Fig.9.8).
`=>` Thus, the energy of the two `color{red}(e_g)` orbitals will increase by `color{red}((3/5) Δ_o)` and that of the three `color{red}(t_(2g))` will decrease by `color{red}((2/5)Δ_o)`.
`=>` The crystal field splitting, `color{red}(Δ_o)`, depends upon the field produced by the ligand and charge on the metal ion.
`=>` Some ligands are able to produce strong fields in which case, the splitting will be large whereas others produce weak fields and consequently result in small splitting of `d`-orbitals.
● In general, ligands can be arranged in a series in the order of increasing field strength as given below :
`color{red}(I^– < Br^– < SCN^– < Cl^– < S2^– < F^– < OH^– < C_2O_4^(2–) < H_2O < NCS^– < edta^(4–) < NH_3 < en < CN^(–) < CO)`
● Such a series is termed as `color{green}("spectrochemical series")`.
● It is an experimentally determined series based on the absorption of light by complexes with different ligands.
`=>` Let us assign electrons in the `color{red}(d)`-orbitals of metal ion in octahedral coordination entities.
● Obviously, the single `color{red}(d)` electron occupies one of the lower energy `color{red}(t_(2g))` orbitals.
● In `color{red}(d^2)` and `color{red}(d^3)` coordination entities, the `color{red}(d)` electrons occupy the `color{red}(t_(2g))` orbitals singly in accordance with the Hund’s rule.
● For `color{red}(d^4)` ions, two possible patterns of electron distribution arise :
(i) the fourth electron could either enter the `color{red}(t_(2g))` level and pair with an existing electron, or
(ii) it could avoid paying the price of the pairing energy by occupying the `color{red}(e_g)` level.
● Which of these possibilities occurs, depends on the relative magnitude of the crystal field splitting, `color{red}(Δ_o)` and the pairing energy, `P` (`P` represents the energy required for electron pairing in a single orbital).
● The two options are :
(i) If `color{red}(Δ_o < P)`, the fourth electron enters one of the `color{red}(e_g)` orbitals giving the configuration `color{red}(t_(2g)^3 e_(g)^1)`. Ligands for which `color{red}(Δ_o < P)` are known as weak field ligands and form high spin complexes.
(ii) If `color{red}(Δ_o > P)`, it becomes more energetically favourable for the fourth electron to occupy a `color{red}(t_(2g))` orbital with configuration `color{red}(t_(2g)^4 e_(g)^0)`. Ligand which produce this effect are known as strong field ligands and form low spin complexes.
`color{red}("Note ")` : Calculations show that `color{red}(d^4)` to `color{red}(d^7)` coordination entities are more stable for strong field as compared to weak field cases.