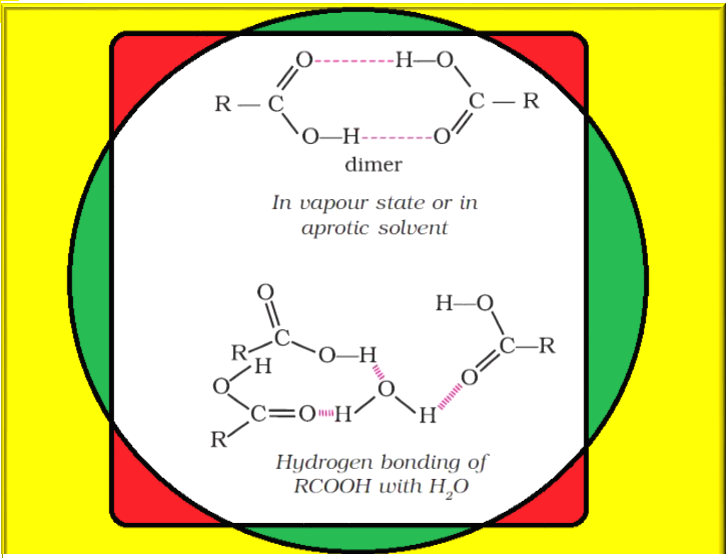
`color{green}(text(Acidity :))`
`color{green}(text(Reactions with Metals and Alkalies :))` The carboxylic acids like alcohols evolve hydrogen with electropositive metals and form salts with alkalies similar to phenols.
● However, unlike phenols they react with weaker bases such as carbonates and hydrogencarbonates to evolve carbon dioxide.
● This reaction is used to detect the presence of carboxyl group in an organic compound.
`color{red}(2R-COOH +2Na → undersettext(Sodium carboxylate) (2R- CO O^(-) Na^+) + H_2)`
`color{red}(R- COOH+NaOH → R - CO O^(-) Na^(+) + H_2O)`
`color{red}(R - COOH+NaHCO_3 → R- CO O^(-) Na^(+) +H_2O+CO_2)`
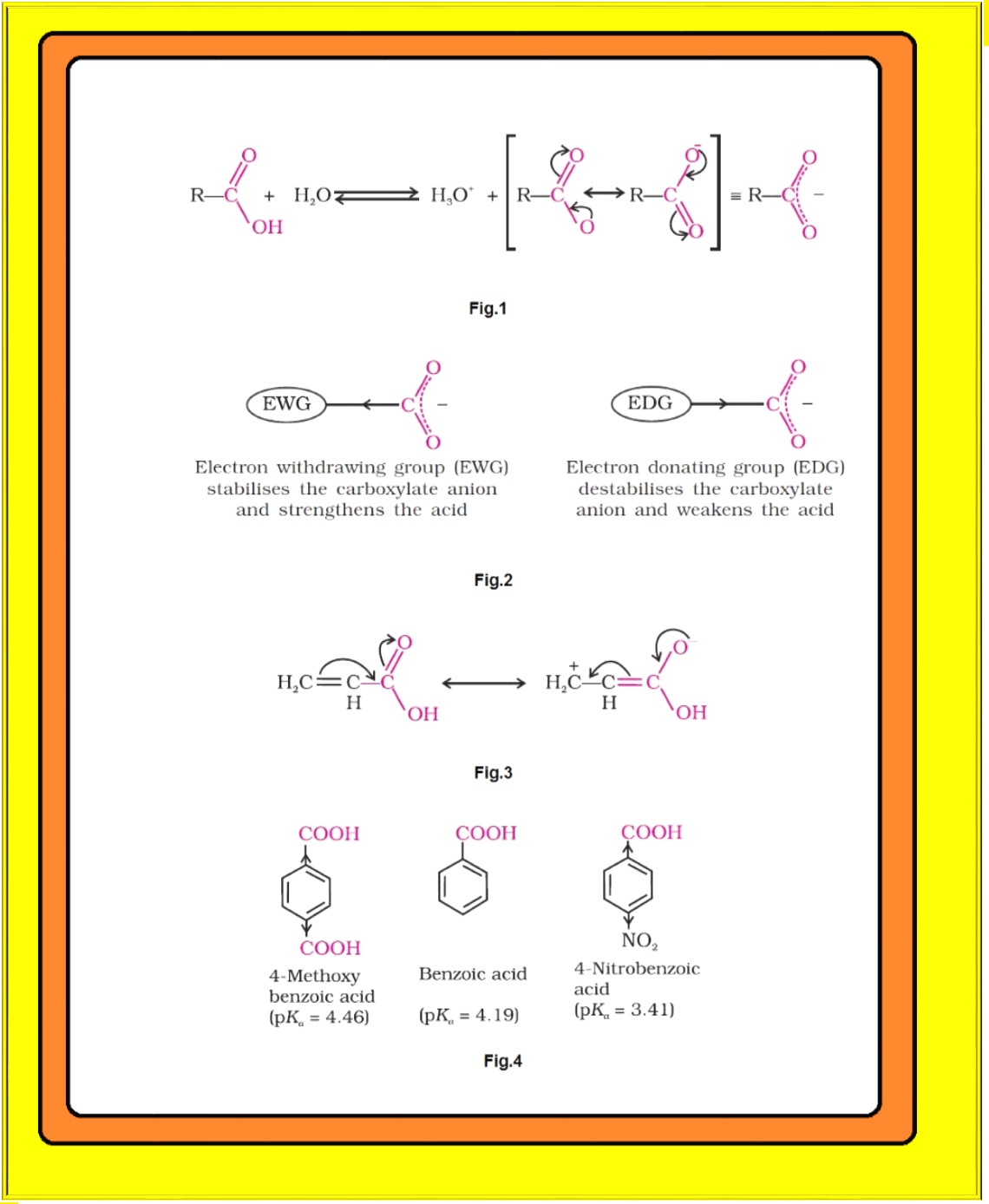
Carboxylic acids dissociate in water to give resonance stabilised carboxylate anions and hydronium ion. See fig.1.
`=>` For the reaction given in fig.1 :
`color{red}(K_(eq) = frac([H_3 overset(+)O] [RCO O^(-)]) ([H_2O][RCOOH]) \ \ \ \ K_a = K_(eq) = ([H_3 overset(+)O] [RCO O^(-)])/([RCOOH]))`
where `color{red}(K_(eq))`, is equilibrium constant and `color{red}(K_a)` is the acid dissociation constant.
`=>` The strength of an acid is generally indicated by its `color{red}(pK_a)` value rather than its `color{red}(K_a)` value.
`color{red}(pK_a = -logK_a)`
`=>` The `color{red}(pK_a)` of hydrochloric acid is `color{red}(-7.0)`, whereas `color{red}(pK_a)` of trifluoroacetic acid (the strongest organic acid), benzoic acid and acetic acid are `color{red}(0.23, 4.19)` and `color{red}(4.76,)` respectively.
`=>` Smaller the `color{red}(pK_a)`, the stronger the acid (the better it is as a proton donor).
`=>` Strong acids have `color{red}(pK_a)` values `color{red}(< 1)`, the acids with `color{red}(pK_a)` values between `1` and `5` are considered to be moderately strong acids, weak acids have `color{red}(pK_a)` values between `5` and `15`, and extremely weak acids have `color{red}(pK_a)` values `color{red}(> 15)`.
`=>` Carboxylic acids are weaker than mineral acids, but they are stronger acids than alcohols and many simple phenols (`color{red}(pK_a)` is `~16` for ethanol and `10` for phenol).
`=>` The conjugate base of carboxylic acid, a carboxylate ion, is stabilised by two equivalent resonance structures in which the negative charge is at the more electronegative oxygen atom.
● The conjugate base of phenol, a phenoxide ion, has non-equivalent resonance structures in which the negative charge is at the less electronegative carbon atom.
● Therefore, resonance in phenoxide ion is not as important as it is in carboxylate ion.
● Further, the negative charge is delocalised over two electronegative oxygen atoms in carboxylate ion whereas it is less effectively delocalised over one oxygen atom and less electronegative carbon atoms in phenoxide ion.
● Thus, the carboxylate ion is more stabilised than phenoxide ion, so carboxylic acids are more acidic than phenols.
`color{green}(text(Effect of Substituents on the Acidity of Carboxylic Acids :))` Substituents may affect the stability of the conjugate base and thus, also affect the acidity of the carboxylic acids.
● Electron withdrawing groups increase the acidity of carboxylic acids by stabilising the conjugate base through delocalisation of the negative charge by inductive and/or resonance effects.
● Electron donating groups decrease the acidity by destabilising the conjugate base. See fig.2.
● The effect of the following groups in increasing acidity order is
`color{red}(Ph < I < Br < Cl < F < CN < NO_2 < CF_3)`
● Thus, the following acids are arranged in order of decreasing acidity (based on `color{red}(pK_a)` values) :
`color{red}(overset(CF_3COOH > C Cl_3COOH > CHCl_2COOH > NO_2CH_2COOH > NC-CH_2COOH > ) ←)`
`color{red}(overset( undersettext(continue)(FCH_2COOH) > ClCH_2COOH > BrCH_2COOH > HCOOH > ClCH_2CH_2COOH > ) ←)`
`color{red}(overset( undersettext(continue)(C_6H_5COOH) > C_6H_5CH_2COOH > CH_3COOH > CH_3CH_2COOH )←)`
`=>` Direct attachment of groups such as phenyl or vinyl to the carboxylic acid, increases the acidity of corresponding carboxylic acid, contrary to the decrease expected due to resonance effect shown in fig.3.
`=>` This is because of greater electronegativity of `color{red}(sp^2)` hybridised carbon to which carboxyl carbon is attached.
● The presence of electron withdrawing group on the phenyl of aromatic carboxylic acid increases their acidity while electron donating groups decrease their acidity. See fig.4
`color{green}(text(Acidity :))`
`color{green}(text(Reactions with Metals and Alkalies :))` The carboxylic acids like alcohols evolve hydrogen with electropositive metals and form salts with alkalies similar to phenols.
● However, unlike phenols they react with weaker bases such as carbonates and hydrogencarbonates to evolve carbon dioxide.
● This reaction is used to detect the presence of carboxyl group in an organic compound.
`color{red}(2R-COOH +2Na → undersettext(Sodium carboxylate) (2R- CO O^(-) Na^+) + H_2)`
`color{red}(R- COOH+NaOH → R - CO O^(-) Na^(+) + H_2O)`
`color{red}(R - COOH+NaHCO_3 → R- CO O^(-) Na^(+) +H_2O+CO_2)`
Carboxylic acids dissociate in water to give resonance stabilised carboxylate anions and hydronium ion. See fig.1.
`=>` For the reaction given in fig.1 :
`color{red}(K_(eq) = frac([H_3 overset(+)O] [RCO O^(-)]) ([H_2O][RCOOH]) \ \ \ \ K_a = K_(eq) = ([H_3 overset(+)O] [RCO O^(-)])/([RCOOH]))`
where `color{red}(K_(eq))`, is equilibrium constant and `color{red}(K_a)` is the acid dissociation constant.
`=>` The strength of an acid is generally indicated by its `color{red}(pK_a)` value rather than its `color{red}(K_a)` value.
`color{red}(pK_a = -logK_a)`
`=>` The `color{red}(pK_a)` of hydrochloric acid is `color{red}(-7.0)`, whereas `color{red}(pK_a)` of trifluoroacetic acid (the strongest organic acid), benzoic acid and acetic acid are `color{red}(0.23, 4.19)` and `color{red}(4.76,)` respectively.
`=>` Smaller the `color{red}(pK_a)`, the stronger the acid (the better it is as a proton donor).
`=>` Strong acids have `color{red}(pK_a)` values `color{red}(< 1)`, the acids with `color{red}(pK_a)` values between `1` and `5` are considered to be moderately strong acids, weak acids have `color{red}(pK_a)` values between `5` and `15`, and extremely weak acids have `color{red}(pK_a)` values `color{red}(> 15)`.
`=>` Carboxylic acids are weaker than mineral acids, but they are stronger acids than alcohols and many simple phenols (`color{red}(pK_a)` is `~16` for ethanol and `10` for phenol).
`=>` The conjugate base of carboxylic acid, a carboxylate ion, is stabilised by two equivalent resonance structures in which the negative charge is at the more electronegative oxygen atom.
● The conjugate base of phenol, a phenoxide ion, has non-equivalent resonance structures in which the negative charge is at the less electronegative carbon atom.
● Therefore, resonance in phenoxide ion is not as important as it is in carboxylate ion.
● Further, the negative charge is delocalised over two electronegative oxygen atoms in carboxylate ion whereas it is less effectively delocalised over one oxygen atom and less electronegative carbon atoms in phenoxide ion.
● Thus, the carboxylate ion is more stabilised than phenoxide ion, so carboxylic acids are more acidic than phenols.
`color{green}(text(Effect of Substituents on the Acidity of Carboxylic Acids :))` Substituents may affect the stability of the conjugate base and thus, also affect the acidity of the carboxylic acids.
● Electron withdrawing groups increase the acidity of carboxylic acids by stabilising the conjugate base through delocalisation of the negative charge by inductive and/or resonance effects.
● Electron donating groups decrease the acidity by destabilising the conjugate base. See fig.2.
● The effect of the following groups in increasing acidity order is
`color{red}(Ph < I < Br < Cl < F < CN < NO_2 < CF_3)`
● Thus, the following acids are arranged in order of decreasing acidity (based on `color{red}(pK_a)` values) :
`color{red}(overset(CF_3COOH > C Cl_3COOH > CHCl_2COOH > NO_2CH_2COOH > NC-CH_2COOH > ) ←)`
`color{red}(overset( undersettext(continue)(FCH_2COOH) > ClCH_2COOH > BrCH_2COOH > HCOOH > ClCH_2CH_2COOH > ) ←)`
`color{red}(overset( undersettext(continue)(C_6H_5COOH) > C_6H_5CH_2COOH > CH_3COOH > CH_3CH_2COOH )←)`
`=>` Direct attachment of groups such as phenyl or vinyl to the carboxylic acid, increases the acidity of corresponding carboxylic acid, contrary to the decrease expected due to resonance effect shown in fig.3.
`=>` This is because of greater electronegativity of `color{red}(sp^2)` hybridised carbon to which carboxyl carbon is attached.
● The presence of electron withdrawing group on the phenyl of aromatic carboxylic acid increases their acidity while electron donating groups decrease their acidity. See fig.4