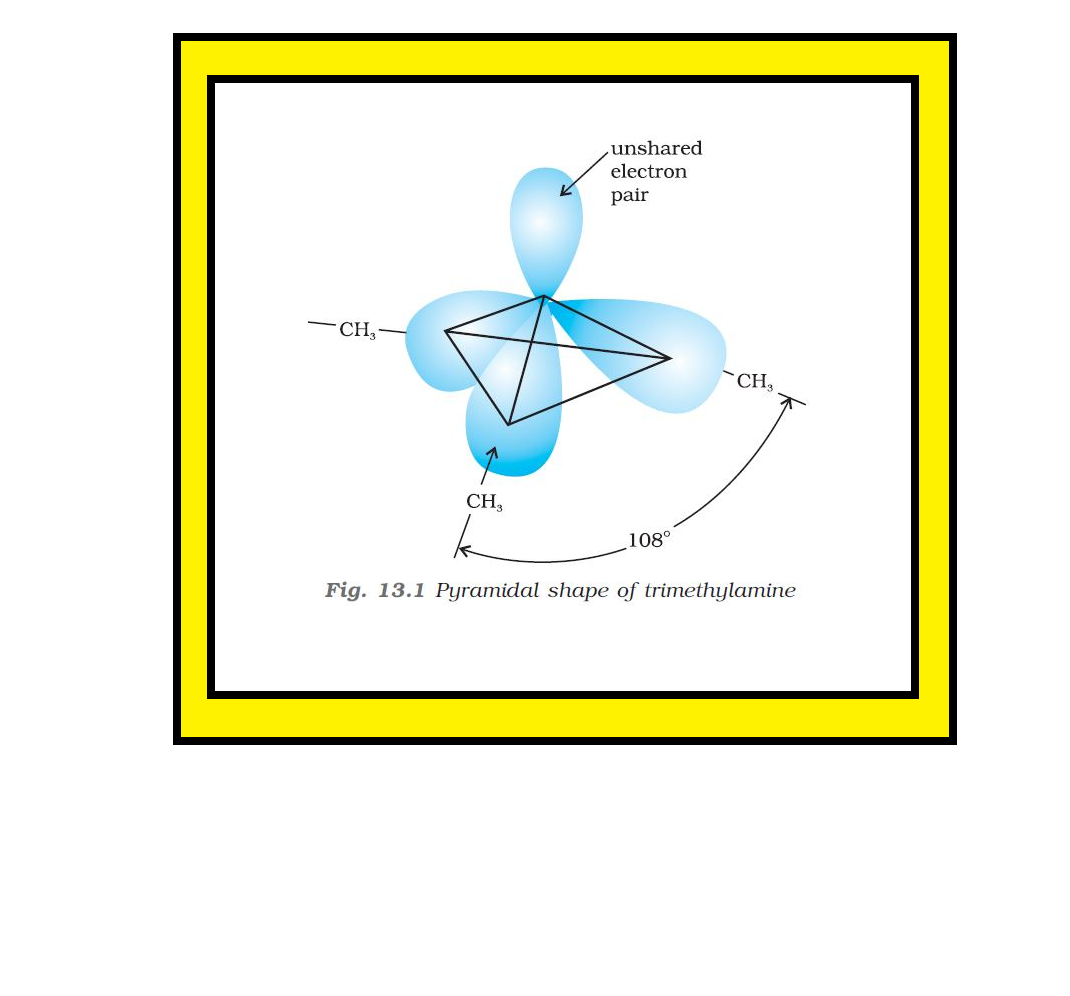
Introduction :
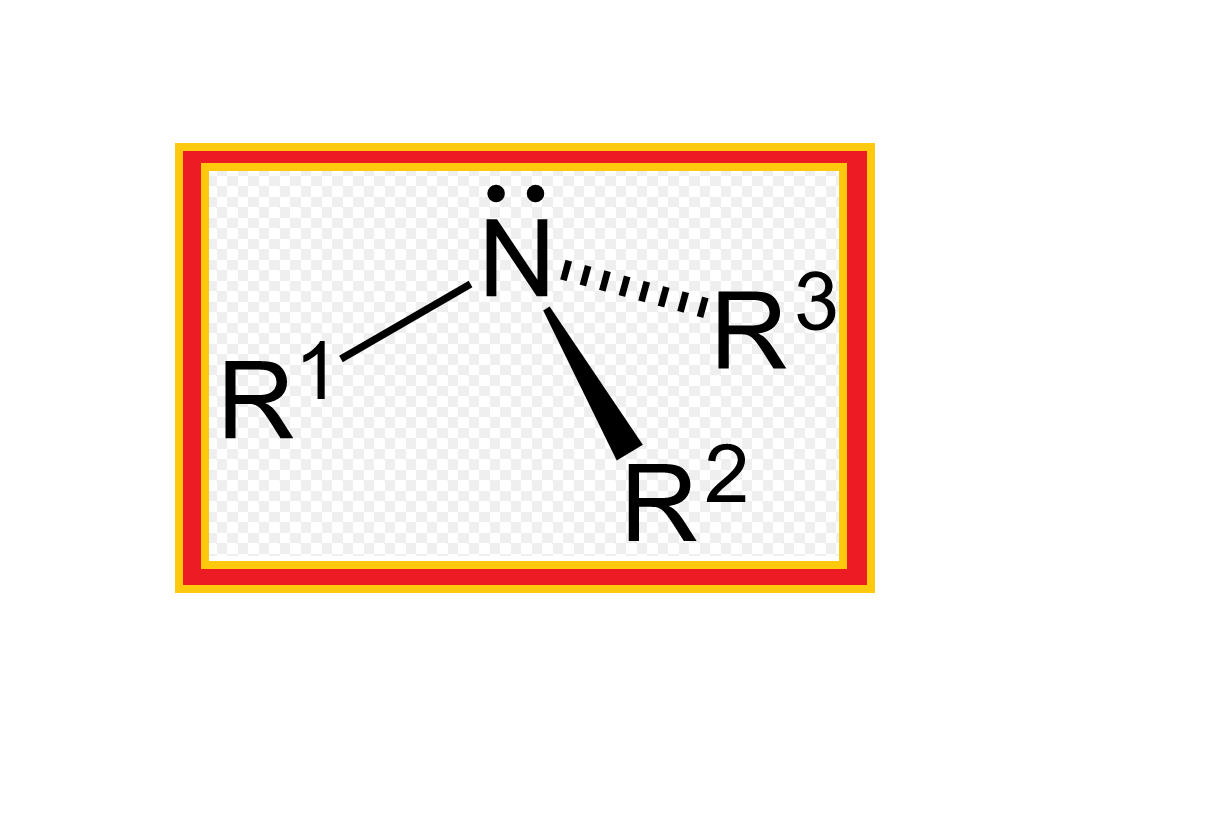
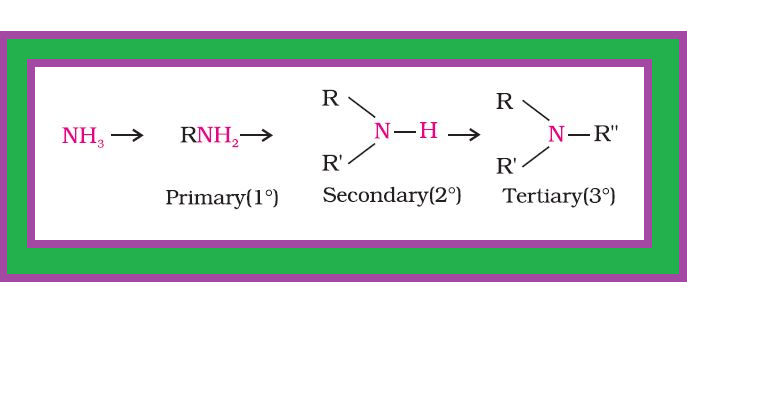
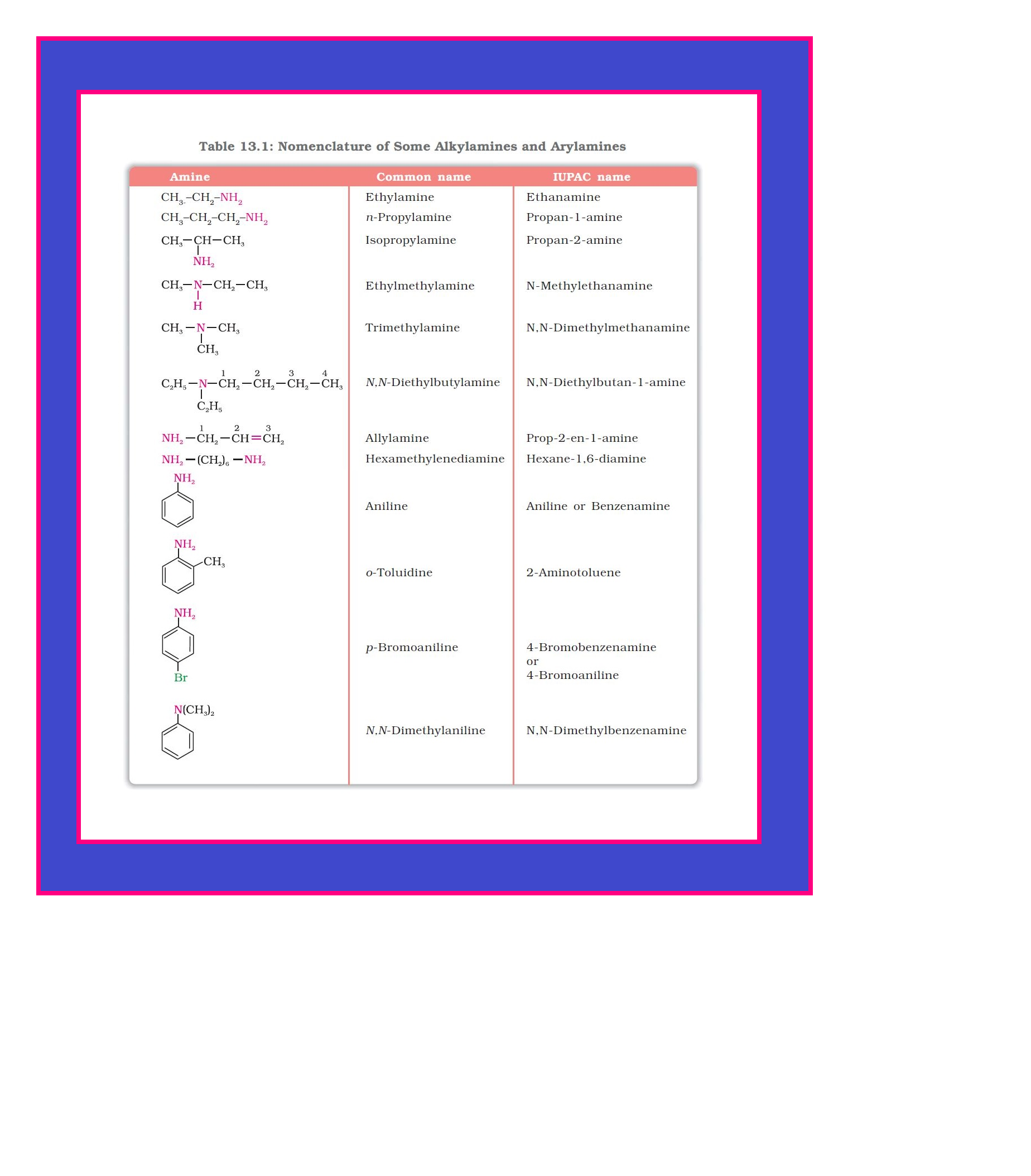
`color{green}(text(Definition ))` : Amines constitute an important class of organic compounds derived by replacing one or more hydrogen atoms of ammonia molecule by alkyl/aryl group(s).
`=>` In nature, they occur among proteins, vitamins, alkaloids and hormones.
`=>` Synthetic examples include polymers, dyestuffs and drugs.
`=>` Two biologically active compounds, namely adrenaline and ephedrine, both containing secondary amino group, are used to increase blood pressure.
`=>` Novocain, a synthetic amino compound, is used as an anaesthetic in dentistry.
● Benadryl, a well known antihistaminic drug also contains tertiary amino group.
● Quaternary ammonium salts are used as surfactants.
● Diazonium salts are intermediates in the preparation of a variety of aromatic compounds including dyes.
`=>` In nature, they occur among proteins, vitamins, alkaloids and hormones.
`=>` Synthetic examples include polymers, dyestuffs and drugs.
`=>` Two biologically active compounds, namely adrenaline and ephedrine, both containing secondary amino group, are used to increase blood pressure.
`=>` Novocain, a synthetic amino compound, is used as an anaesthetic in dentistry.
● Benadryl, a well known antihistaminic drug also contains tertiary amino group.
● Quaternary ammonium salts are used as surfactants.
● Diazonium salts are intermediates in the preparation of a variety of aromatic compounds including dyes.