Diazonium Salts :
`=>` The diazonium salts have the general formula `color{red}(R overset(+)N_2 overset(-)X)` where `color{red}(R)` stands for an aryl group and `color{red}(overset(-)X)` ion may be `color{red}(Cl^(-), Br^(-), HSO_4^(-), BF_4^(-))`, etc.
`=>` They are named by suffixing diazonium to the name of the parent hydrocarbon from which they are formed, followed by the name of anion such as chloride, hydrogensulphate, etc.
`=>` The `color{red}(overset(+)N_2)` group is called diazonium group.
● For example, `color{red}(C_6H_5 overset(+)N_2 overset(-)Cl)` is named as benzenediazonium chloride and `color{red}(C_6H_5N_2^(+)HSO_4^(-))` is known as benzenediazonium hydrogensulphate.
`=>` Primary aliphatic amines form highly unstable alkyldiazonium salts.
`=>` Primary aromatic amines form arenediazonium salts which are stable for a short time in solution at low temperatures (`273-278 K`).
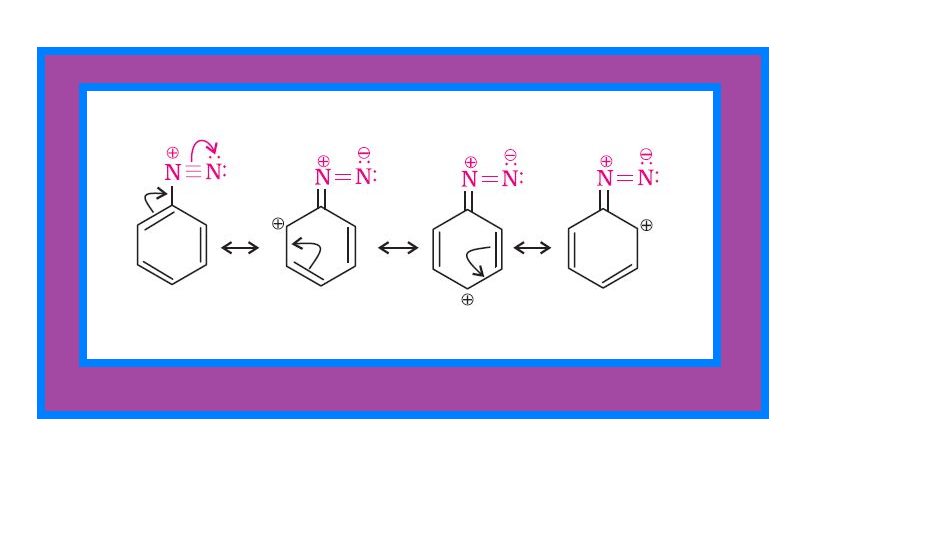
`=>` The stability of arenediazonium ion is explained on the basis of resonance. See fig.
`=>` They are named by suffixing diazonium to the name of the parent hydrocarbon from which they are formed, followed by the name of anion such as chloride, hydrogensulphate, etc.
`=>` The `color{red}(overset(+)N_2)` group is called diazonium group.
● For example, `color{red}(C_6H_5 overset(+)N_2 overset(-)Cl)` is named as benzenediazonium chloride and `color{red}(C_6H_5N_2^(+)HSO_4^(-))` is known as benzenediazonium hydrogensulphate.
`=>` Primary aliphatic amines form highly unstable alkyldiazonium salts.
`=>` Primary aromatic amines form arenediazonium salts which are stable for a short time in solution at low temperatures (`273-278 K`).
`=>` The stability of arenediazonium ion is explained on the basis of resonance. See fig.