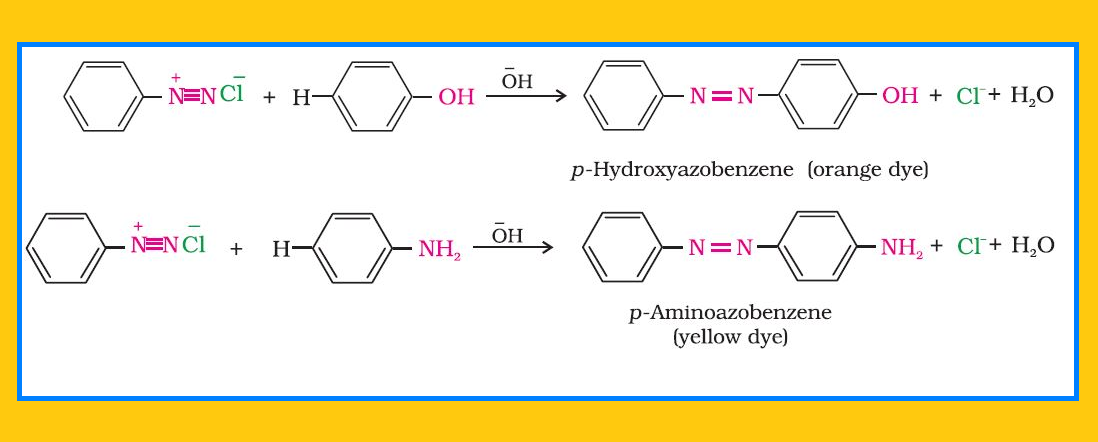
Reactions involving displacement of nitrogen :
`=>` Diazonium group being a very good leaving group, is substituted by other groups such as `color{red}(Cl^–, Br^–, I^–, CN^–)` and `color{red}(OH^–)` which displace nitrogen from the aromatic ring. The nitrogen formed escapes from the reaction mixture as a gas.
(i) `color{green}(text(Replacement by halide or cyanide ion ))` : The `color{red}(Cl^–, Br^–)` and `color{red}(CN^–)` nucleophiles can easily be introduced in the benzene ring in the presence of `color{red}(Cu(I))` ion. This reaction is called `color{green}(text(Sandmeyer reaction))`.
`color{red}(Ar overset(+)N_2 overset(-)X overset(CuCl//HCl)→ ArCl +N_2)`
`color{red}(Ar overset(+)N_2 overset(-)X overset(CuBr//HBr)→ ArBr+N_2)`
`color{red}(Ar overset(+)N_2 overset(-)X overset(CuCN//KCN)→ ArCN +N_2)`
`=>` Alternatively, chlorine or bromine can also be introduced in the benzene ring by treating the diazonium salt solution with corresponding halogen acid in the presence of copper powder. This is referred as `color{green}(text(Gatterman reaction))`.
`color{red}(Aroverset(+)N_2 overset(-)X overset(Cu//HCl)→ ArCl+N_2+CuX)`
`color{red}(Aroverset(+)N_2 overset(-)X overset(Cu//HBr)→ ArBr +N_2+CuX)`
`=>` The yield in Sandmeyer reaction is found to be better than Gattermann reaction.
(ii) `color{green}(text(Replacement by iodide ion ))` : Iodine is not easily introduced into the benzene ring directly, but, when the diazonium salt solution is treated with potassium iodide, iodobenzene is formed.
`color{red}(Aroverset(+)N_2 C overset(-)l +KI → ArI+KCl+N_2)`
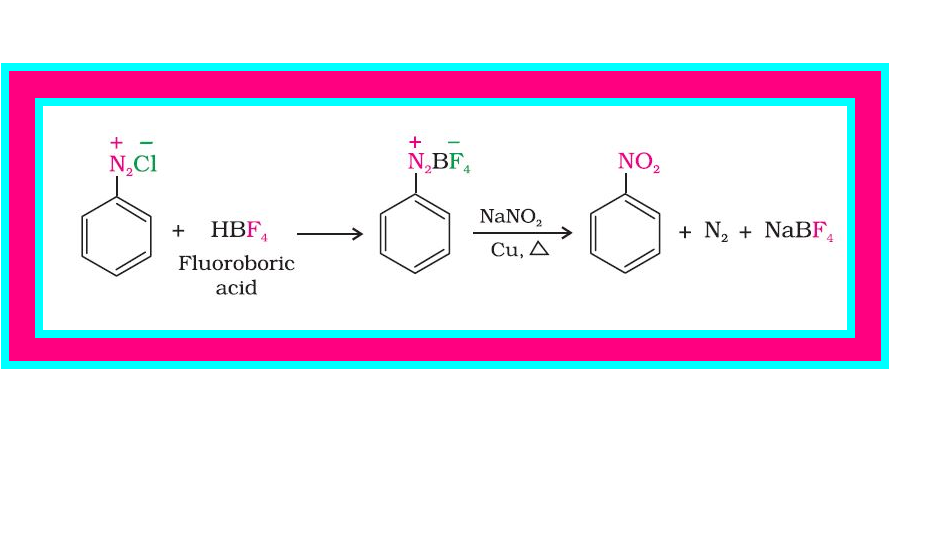
(iii) `color{green}(text(Replacement by fluoride ion ))` : When arenediazonium chloride is treated with fluoroboric acid, arene diazonium fluoroborate is precipitated which on heating decomposes to yield aryl fluoride.
`color{red}(Aroverset(+)N_2 C overset(-)l +HBF_4 → Ar - overset(+)N_2 B overset(-)F_4 overset(Delta)→ Ar-F +BF_3+N_2)`
(iv) `color{green}(text(Replacement by H))` : Certain mild reducing agents like hypophosphorous acid (phosphinic acid) or ethanol reduce diazonium salts to arenes and themselves get oxidised to phosphorous acid and ethanal, respectively.
`color{red}(Aroverset(+)N_2 C overset(-)l +H_3PO_2+H_2O →ArH+N_2+H_3PO_3+HCl)`
`color{red}(Ar overset(+)N_2 C overset(-)l +CH_3CH_2OH → ArH +N_2+CH_3CHO+HCl)`
(v) `color{green}(text(Replacement by hydroxyl group ))` : If the temperature of the diazonium salt solution is allowed to rise upto `283 K`, the salt gets hydrolysed to phenol.
`color{red}(Ar overset(+)N_2 C overset(-)l +H_2O → ArOH +N_2 +HCl)`
(vi) `color{green}(text(Replacement by))` `color{red}(–NO_2)` `color{green}(text(group ))` : When diazonium fluoroborate is heated with aqueous sodium nitrite solution in the presence of copper, the diazonium group is replaced by `color{red}(–NO_2)` group. See fig.
(i) `color{green}(text(Replacement by halide or cyanide ion ))` : The `color{red}(Cl^–, Br^–)` and `color{red}(CN^–)` nucleophiles can easily be introduced in the benzene ring in the presence of `color{red}(Cu(I))` ion. This reaction is called `color{green}(text(Sandmeyer reaction))`.
`color{red}(Ar overset(+)N_2 overset(-)X overset(CuCl//HCl)→ ArCl +N_2)`
`color{red}(Ar overset(+)N_2 overset(-)X overset(CuBr//HBr)→ ArBr+N_2)`
`color{red}(Ar overset(+)N_2 overset(-)X overset(CuCN//KCN)→ ArCN +N_2)`
`=>` Alternatively, chlorine or bromine can also be introduced in the benzene ring by treating the diazonium salt solution with corresponding halogen acid in the presence of copper powder. This is referred as `color{green}(text(Gatterman reaction))`.
`color{red}(Aroverset(+)N_2 overset(-)X overset(Cu//HCl)→ ArCl+N_2+CuX)`
`color{red}(Aroverset(+)N_2 overset(-)X overset(Cu//HBr)→ ArBr +N_2+CuX)`
`=>` The yield in Sandmeyer reaction is found to be better than Gattermann reaction.
(ii) `color{green}(text(Replacement by iodide ion ))` : Iodine is not easily introduced into the benzene ring directly, but, when the diazonium salt solution is treated with potassium iodide, iodobenzene is formed.
`color{red}(Aroverset(+)N_2 C overset(-)l +KI → ArI+KCl+N_2)`
(iii) `color{green}(text(Replacement by fluoride ion ))` : When arenediazonium chloride is treated with fluoroboric acid, arene diazonium fluoroborate is precipitated which on heating decomposes to yield aryl fluoride.
`color{red}(Aroverset(+)N_2 C overset(-)l +HBF_4 → Ar - overset(+)N_2 B overset(-)F_4 overset(Delta)→ Ar-F +BF_3+N_2)`
(iv) `color{green}(text(Replacement by H))` : Certain mild reducing agents like hypophosphorous acid (phosphinic acid) or ethanol reduce diazonium salts to arenes and themselves get oxidised to phosphorous acid and ethanal, respectively.
`color{red}(Aroverset(+)N_2 C overset(-)l +H_3PO_2+H_2O →ArH+N_2+H_3PO_3+HCl)`
`color{red}(Ar overset(+)N_2 C overset(-)l +CH_3CH_2OH → ArH +N_2+CH_3CHO+HCl)`
(v) `color{green}(text(Replacement by hydroxyl group ))` : If the temperature of the diazonium salt solution is allowed to rise upto `283 K`, the salt gets hydrolysed to phenol.
`color{red}(Ar overset(+)N_2 C overset(-)l +H_2O → ArOH +N_2 +HCl)`
(vi) `color{green}(text(Replacement by))` `color{red}(–NO_2)` `color{green}(text(group ))` : When diazonium fluoroborate is heated with aqueous sodium nitrite solution in the presence of copper, the diazonium group is replaced by `color{red}(–NO_2)` group. See fig.