Amino Acids :
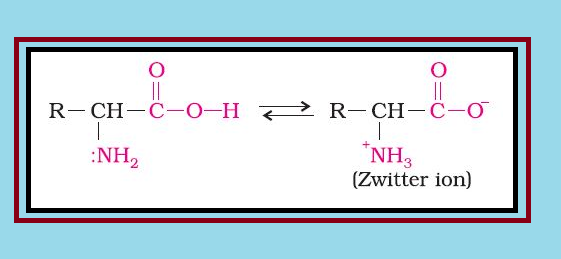
`=>` Amino acids contain amino `color{red}(–NH_2)` and carboxyl `color{red}(–COOH)` functional groups.
`=>` Depending upon the relative position of amino group with respect to carboxyl group, the amino acids can be classified as `color{red}(α, β, γ, δ)` and so on.
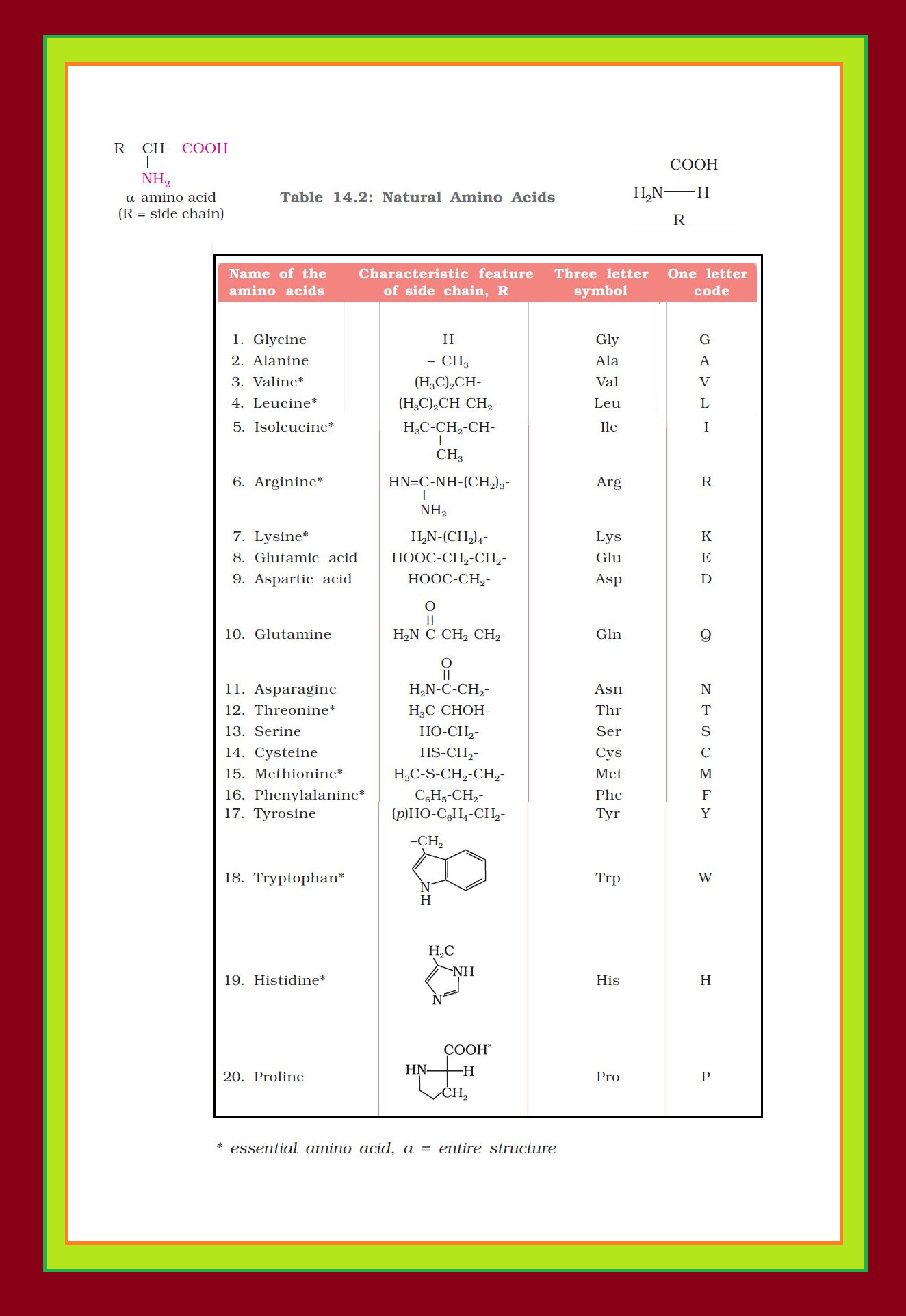
`=>` Only `color{red}(α)`-amino acids are obtained on hydrolysis of proteins. They may contain other functional groups also. See fig.1.
`=>` All `color{red}(α)`-amino acids have trivial names, which usually reflect the property of that compound or its source.
`=>` Glycine is so named since it has sweet taste (in Greek glykos means sweet) and tyrosine was first obtained from cheese (in Greek, tyros means cheese).
`=>` Amino acids are generally represented by a three letter symbol, sometimes one letter symbol is also used.
`=>` Structures of some commonly occurring amino acids along with their 3-letter and 1-letter symbols are given in Table 14.2.
`=>` Depending upon the relative position of amino group with respect to carboxyl group, the amino acids can be classified as `color{red}(α, β, γ, δ)` and so on.
`=>` Only `color{red}(α)`-amino acids are obtained on hydrolysis of proteins. They may contain other functional groups also. See fig.1.
`=>` All `color{red}(α)`-amino acids have trivial names, which usually reflect the property of that compound or its source.
`=>` Glycine is so named since it has sweet taste (in Greek glykos means sweet) and tyrosine was first obtained from cheese (in Greek, tyros means cheese).
`=>` Amino acids are generally represented by a three letter symbol, sometimes one letter symbol is also used.
`=>` Structures of some commonly occurring amino acids along with their 3-letter and 1-letter symbols are given in Table 14.2.