`=>` A large number of polymer applications in different fields depend on their unique mechanical properties like tensile strength, elasticity, toughness, etc.
`=>` These mechanical properties are governed by intermolecular forces, e.g., van der Waals forces and hydrogen bonds, present in the polymer. These forces also bind the polymer chains.
`=>` Under this category, the polymers are classified into the following four sub groups on the basis of magnitude of intermolecular forces present in them.
(i) `color{green}("Elastomers ")` : These are rubber-like solids with elastic properties.
● In these elastomeric polymers, the polymer chains are held together by the weakest intermolecular forces.
● These weak binding forces permit the polymer to be stretched.
● A few ‘crosslinks’ are introduced in between the chains, which help the polymer to retract to its original position after the force is released as in vulcanised rubber.
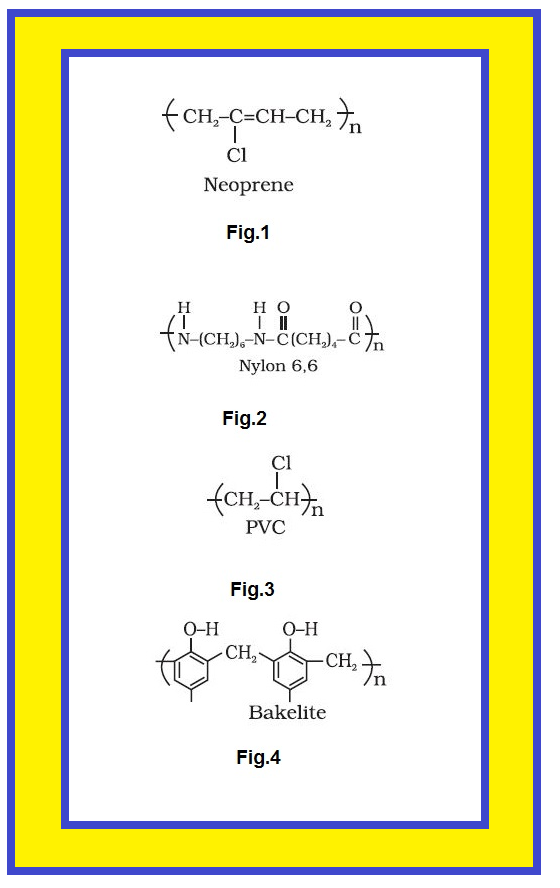
● The examples are buna-S, buna-N, neoprene(fig.1), etc.
(ii) `color{green}("Fibres ")` : Fibres are the thread forming solids which possess high tensile strength and high modulus.
● These characteristics can be attributed to the strong intermolecular forces like hydrogen bonding.
● These strong forces also lead to close packing of chains and thus impart crystalline nature.
● The examples are polyamides (nylon 6, 6)(fig.2), polyesters (terylene), etc.
(iii) `color{green}("Thermoplastic Polymers ")` : These are the linear or slightly branched long chain molecules capable of repeatedly softening on heating and hardening on cooling.
● These polymers possess intermolecular forces of attraction intermediate between elastomers and fibres.
● Some common thermoplastics are polythene, polystyrene, polyvinyls(fig.3.), etc.
(iv) `color{green}("Thermosetting Polymers ")` : These polymers are cross linked or heavily branched molecules, which on heating undergo extensive cross linking in moulds and again become infusible.
● These cannot be reused.
● Some common examples are bakelite, urea-formaldelyde resins, etc.
`=>` A large number of polymer applications in different fields depend on their unique mechanical properties like tensile strength, elasticity, toughness, etc.
`=>` These mechanical properties are governed by intermolecular forces, e.g., van der Waals forces and hydrogen bonds, present in the polymer. These forces also bind the polymer chains.
`=>` Under this category, the polymers are classified into the following four sub groups on the basis of magnitude of intermolecular forces present in them.
(i) `color{green}("Elastomers ")` : These are rubber-like solids with elastic properties.
● In these elastomeric polymers, the polymer chains are held together by the weakest intermolecular forces.
● These weak binding forces permit the polymer to be stretched.
● A few ‘crosslinks’ are introduced in between the chains, which help the polymer to retract to its original position after the force is released as in vulcanised rubber.
● The examples are buna-S, buna-N, neoprene(fig.1), etc.
(ii) `color{green}("Fibres ")` : Fibres are the thread forming solids which possess high tensile strength and high modulus.
● These characteristics can be attributed to the strong intermolecular forces like hydrogen bonding.
● These strong forces also lead to close packing of chains and thus impart crystalline nature.
● The examples are polyamides (nylon 6, 6)(fig.2), polyesters (terylene), etc.
(iii) `color{green}("Thermoplastic Polymers ")` : These are the linear or slightly branched long chain molecules capable of repeatedly softening on heating and hardening on cooling.
● These polymers possess intermolecular forces of attraction intermediate between elastomers and fibres.
● Some common thermoplastics are polythene, polystyrene, polyvinyls(fig.3.), etc.
(iv) `color{green}("Thermosetting Polymers ")` : These polymers are cross linked or heavily branched molecules, which on heating undergo extensive cross linking in moulds and again become infusible.
● These cannot be reused.
● Some common examples are bakelite, urea-formaldelyde resins, etc.