Free radical mechanism :
`=>` A variety of alkenes or dienes and their derivatives are polymerised in the presence of a free radical generating initiator (catalyst) like benzoyl peroxide, acetyl peroxide, tert-butyl peroxide, etc.
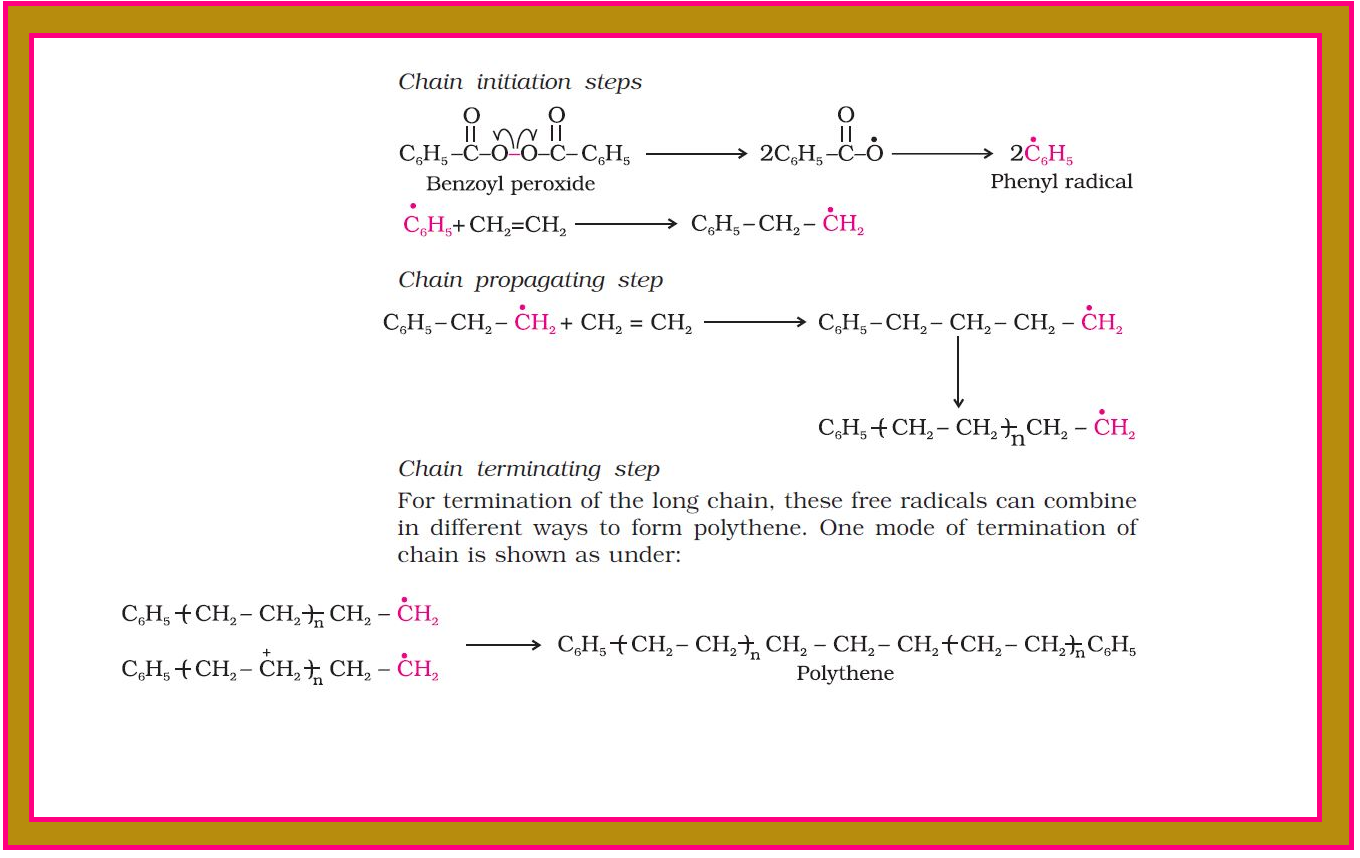
`color{red}("Example ")` : The polymerisation of ethene to polythene consists of heating or exposing to light a mixture of ethene with a small amount of benzoyl peroxide initiator.
● The process starts with the addition of phenyl free radical formed by the peroxide to the ethene double bond thus generating a new and larger free radical. This step is called chain initiating step.
● As this radical reacts with another molecule of ethene, another bigger sized radical is formed. The repetition of this sequence with new and bigger radicals carries the reaction forward and the step is termed as chain propagating step.
● At some stage the product radical thus formed reacts with another radical to form the polymerised product. This step is called the chain terminating step.
● The sequence of steps may be depicted as shown in fig.
`color{red}("Example ")` : The polymerisation of ethene to polythene consists of heating or exposing to light a mixture of ethene with a small amount of benzoyl peroxide initiator.
● The process starts with the addition of phenyl free radical formed by the peroxide to the ethene double bond thus generating a new and larger free radical. This step is called chain initiating step.
● As this radical reacts with another molecule of ethene, another bigger sized radical is formed. The repetition of this sequence with new and bigger radicals carries the reaction forward and the step is termed as chain propagating step.
● At some stage the product radical thus formed reacts with another radical to form the polymerised product. This step is called the chain terminating step.
● The sequence of steps may be depicted as shown in fig.