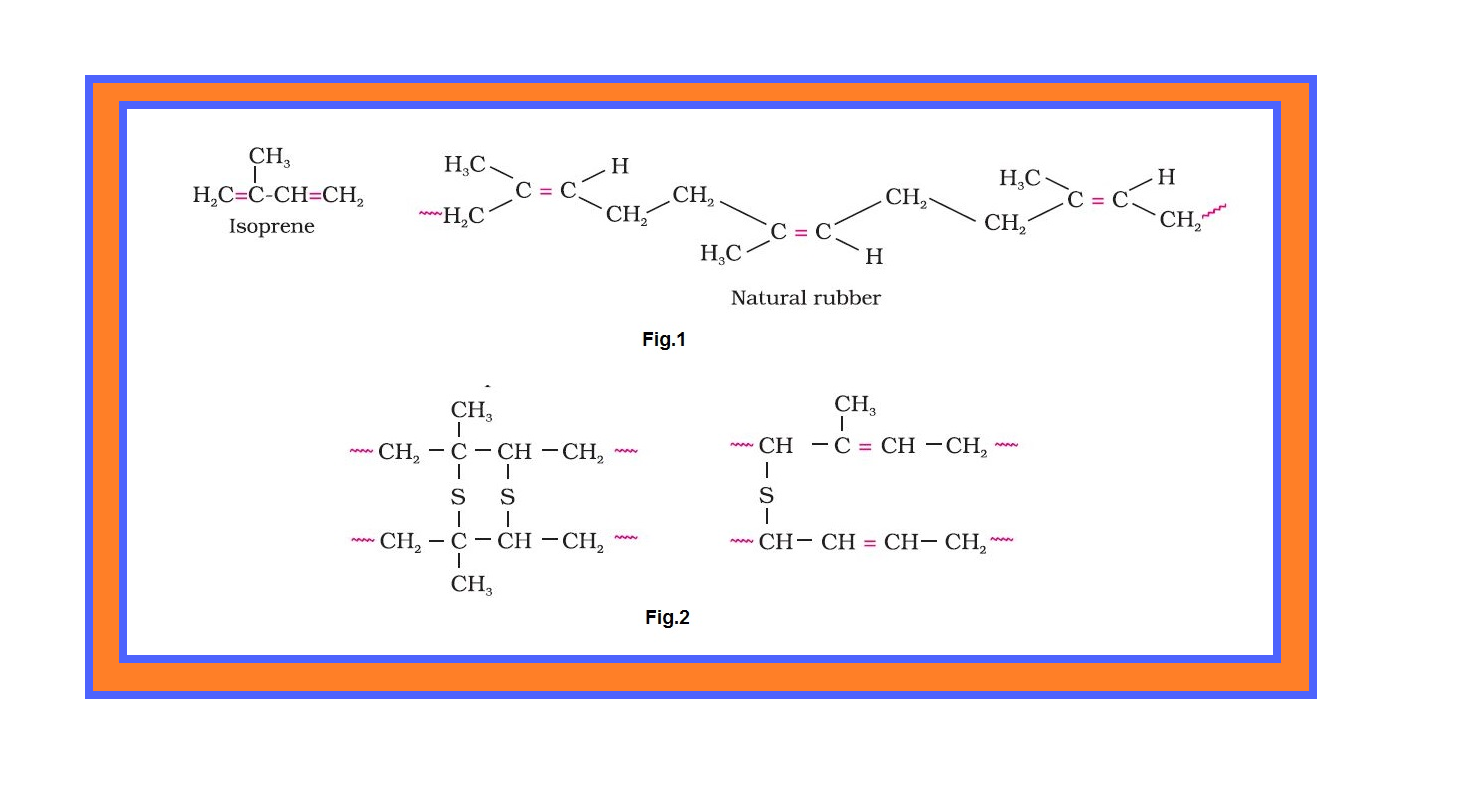
Copolymerisation :
`color{green}("Copolymerisation ")` : It is a polymerisation reaction in which a mixture of more than one monomeric species is allowed to polymerise and form a copolymer.
`=>` The copolymer can be made not only by chain growth polymerisation but by step growth polymerisation also.
`=>` It contains multiple units of each monomer used in the same polymeric chain.
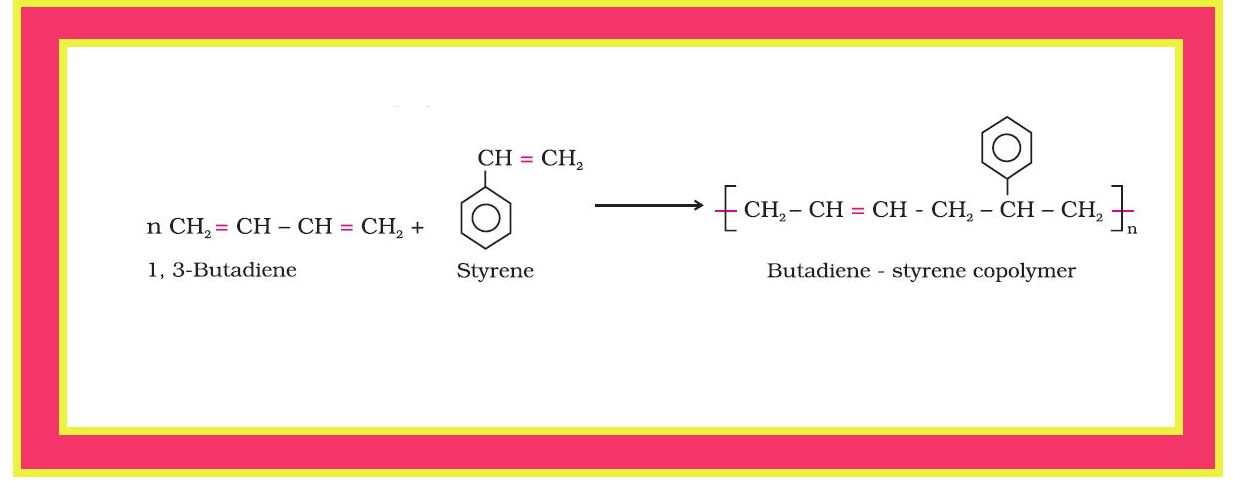
●`color{red}("Example ")` : A mixture of 1, 3 – butadiene and styrene can form a copolymer.
`=>` Copolymers have properties quite different from homopolymers.
●`color{red}("Example ")` : Butadiene - styrene copolymer is quite tough and is a good substitute for natural rubber. It is used for the manufacture of autotyres, floortiles, footwear components, cable insulation, etc.
`=>` The copolymer can be made not only by chain growth polymerisation but by step growth polymerisation also.
`=>` It contains multiple units of each monomer used in the same polymeric chain.
●`color{red}("Example ")` : A mixture of 1, 3 – butadiene and styrene can form a copolymer.
`=>` Copolymers have properties quite different from homopolymers.
●`color{red}("Example ")` : Butadiene - styrene copolymer is quite tough and is a good substitute for natural rubber. It is used for the manufacture of autotyres, floortiles, footwear components, cable insulation, etc.