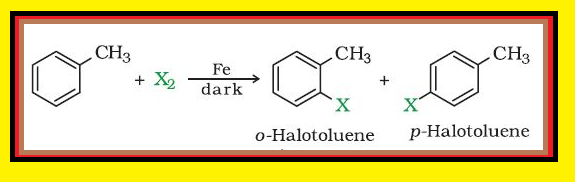
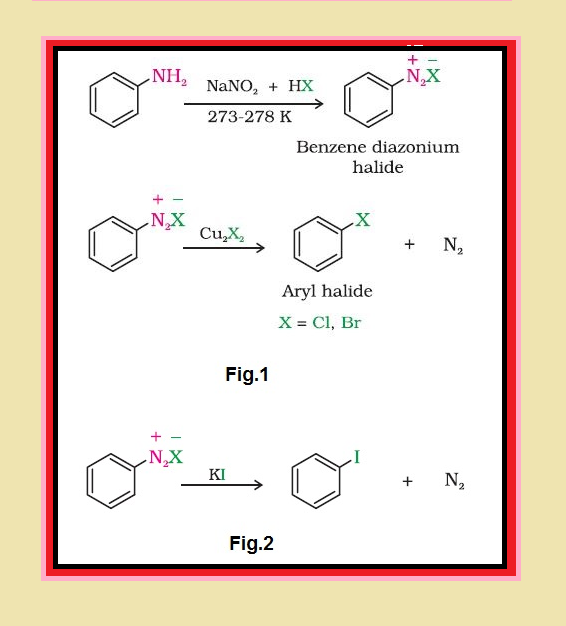
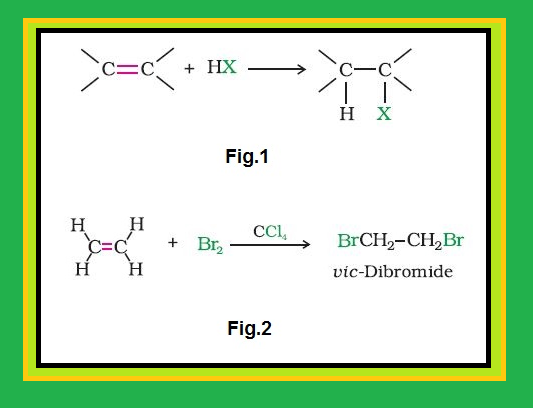
Nature of `C-X` Bond :
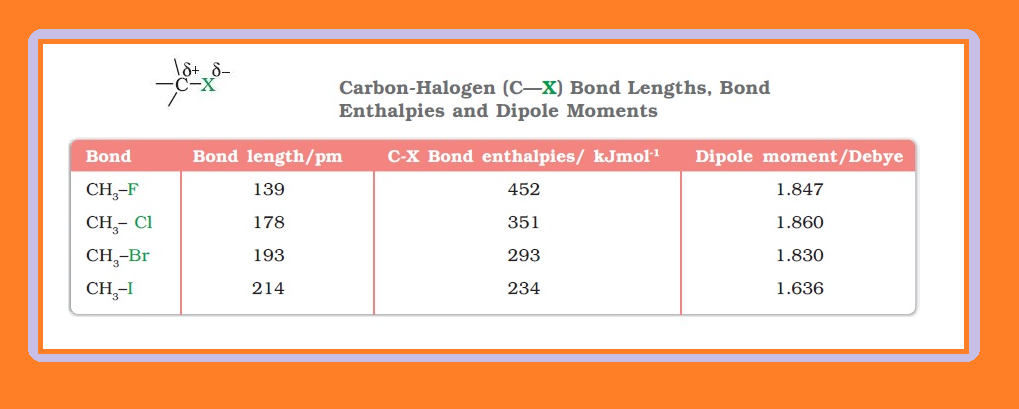
`=>` Since halogen atoms are more electronegative than carbon, the carbon-halogen bond of alkyl halide is polarised; the carbon atom bears a partial positive charge whereas the halogen atom bears a partial negative charge as shown in fig.
`=>` Since the size of halogen atom increases as we go down the group in the periodic table, fluorine atom is the smallest and iodine atom, the largest.
● Consequently the carbon-halogen bond length also increases from `color{red}(C—F)` to `color{red}(C—l)`.
`=>` Some typical bond lengths, bond enthalpies and dipole moments are given in Table 10.2.
`=>` Since the size of halogen atom increases as we go down the group in the periodic table, fluorine atom is the smallest and iodine atom, the largest.
● Consequently the carbon-halogen bond length also increases from `color{red}(C—F)` to `color{red}(C—l)`.
`=>` Some typical bond lengths, bond enthalpies and dipole moments are given in Table 10.2.