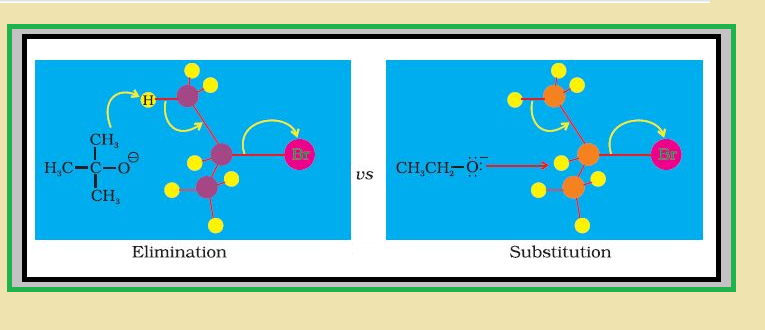
Elimination reactions :
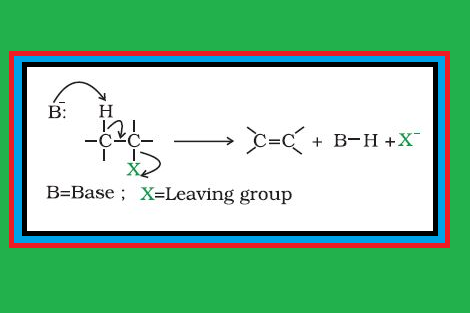
`=>` When a haloalkane with `color{red}(β)`-hydrogen atom is heated with alcoholic solution of potassium hydroxide, there is elimination of hydrogen atom from `color{red}(β)`-carbon and a halogen atom from the `color{red}(α)`-carbon atom.
● As a result, an alkene is formed as a product.
● Since `color{red}(β)`-hydrogen atom is involved in elimination, it is often called `color{red}(β)`-elimination. See fig.
`=>` If there is possibility of formation of more than one alkene due to the availability of more than one `color{red}(α)`-hydrogen atoms, usually one alkene is formed as the major product.
● Alexander Zaitsev (also pronounced as Saytzeff) in 1875 formulated a rule summarised as “In dehydrohalogenation reactions, the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms.”
● Thus, 2-bromopentane gives pent-2-ene as the major product.
`color{red}(undersettext{pent -2- ene (81 %) } (H_3C-CH_2-CH= CH- CH_3) overset{ overset(-) OH } ← undersettext(2- Bromopentane) ( H_3C - CH_2-CH_2- overset( overset(Br)(|))CH - underset(underset(H)(|))CH_2) overset{ overset(-)OH } → undersettext{Pent -1- ene (19 % ) } (H_3C- CH_2-CH_2-CH = CH_2))`
● As a result, an alkene is formed as a product.
● Since `color{red}(β)`-hydrogen atom is involved in elimination, it is often called `color{red}(β)`-elimination. See fig.
`=>` If there is possibility of formation of more than one alkene due to the availability of more than one `color{red}(α)`-hydrogen atoms, usually one alkene is formed as the major product.
● Alexander Zaitsev (also pronounced as Saytzeff) in 1875 formulated a rule summarised as “In dehydrohalogenation reactions, the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms.”
● Thus, 2-bromopentane gives pent-2-ene as the major product.
`color{red}(undersettext{pent -2- ene (81 %) } (H_3C-CH_2-CH= CH- CH_3) overset{ overset(-) OH } ← undersettext(2- Bromopentane) ( H_3C - CH_2-CH_2- overset( overset(Br)(|))CH - underset(underset(H)(|))CH_2) overset{ overset(-)OH } → undersettext{Pent -1- ene (19 % ) } (H_3C- CH_2-CH_2-CH = CH_2))`