Nucleophilic substitution :
`=>` Aryl halides are extremely less reactive towards nucleophilic substitution reactions due to the following reasons :
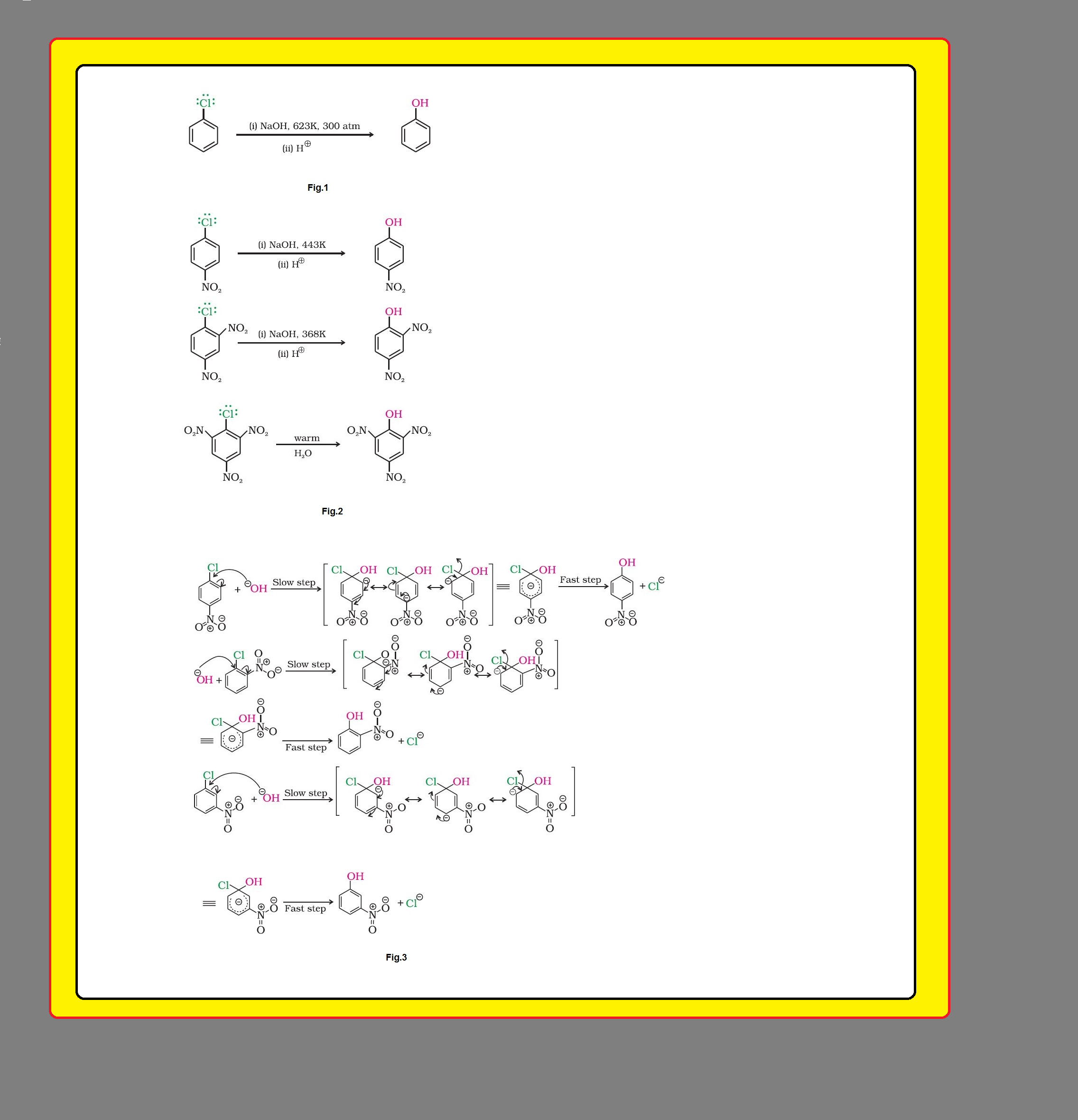
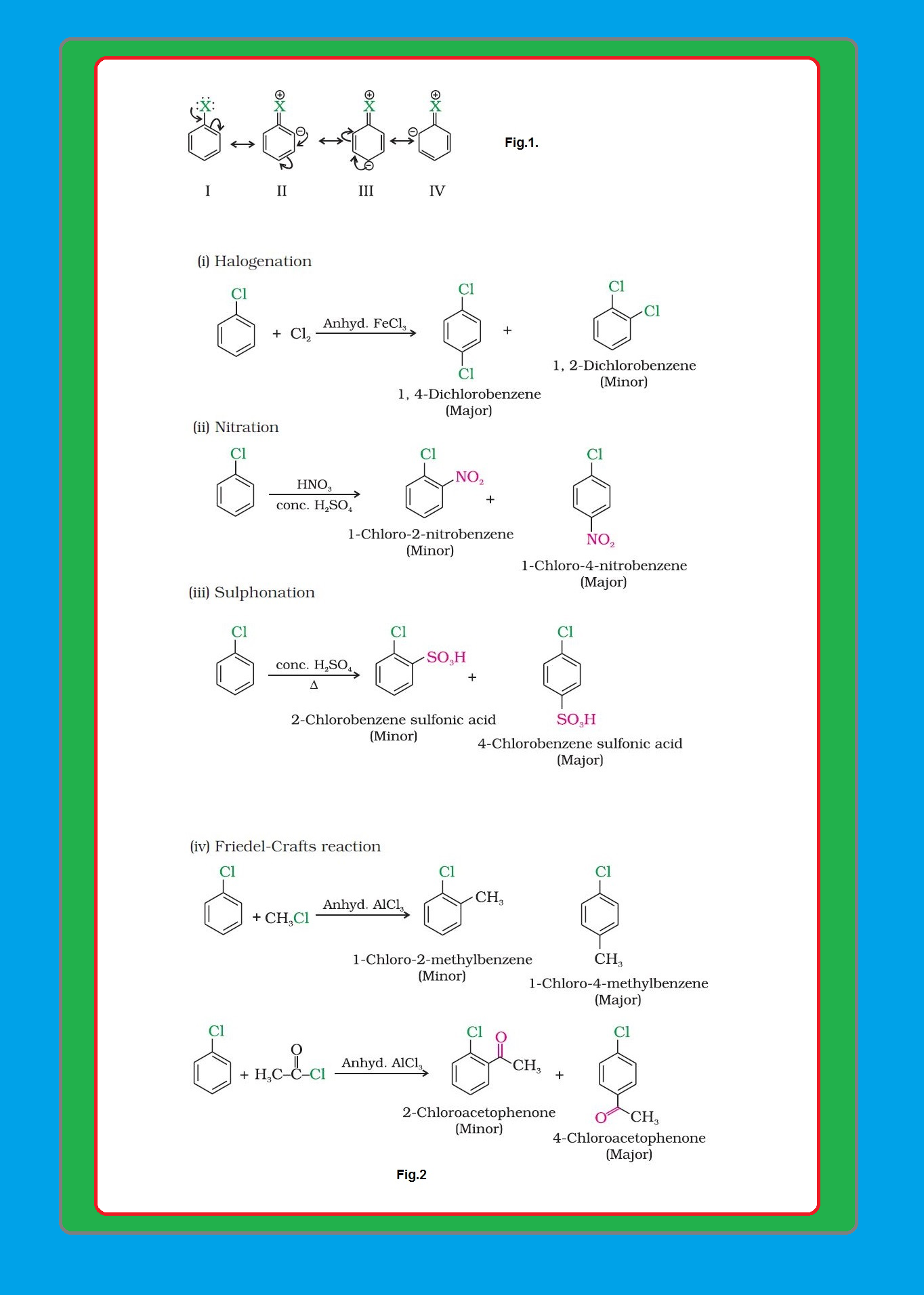
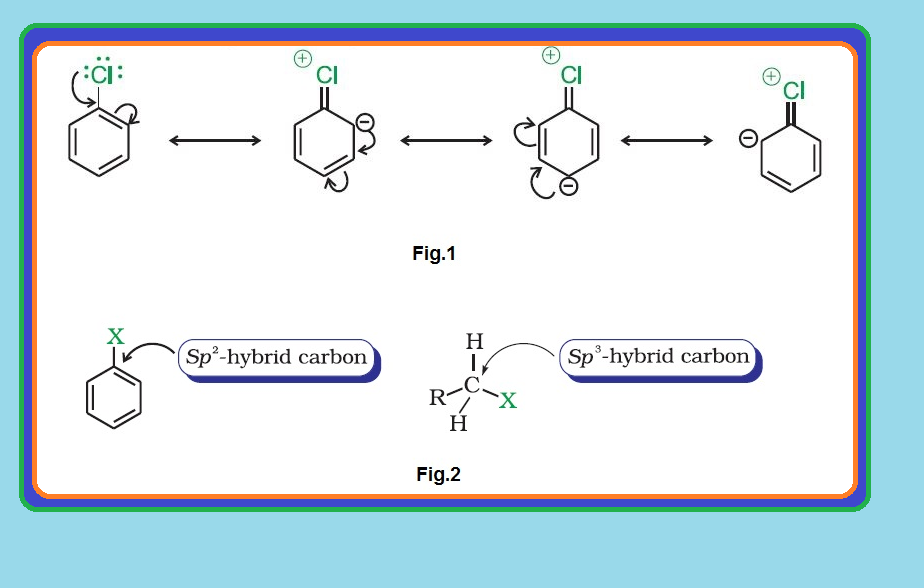
(i) `color{green}("Resonance effect ")` : In haloarenes, the electron pairs on halogen atom are in conjugation with `color{red}(π)`-electrons of the ring and the resonating structures as shown in fig.1 are possible.
`=>` `color{red}(C—Cl)` bond acquires a partial double bond character due to resonance.
● Therefore, the bond cleavage in haloarene is difficult than haloalkane and therefore, they are less reactive towards nucleophilic substitution reaction.
(ii) `color{green}("Difference in hybridisation of carbon atom in C—X bond")` : In haloalkane, the carbon atom attached to halogen is `color{red}(sp^3)` hybridised while in case of haloarene, the carbon atom attached to halogen is `color{red}(sp^2)`-hybridised. See fig.2.
`=>` The `color{red}(sp^2)` hybridised carbon with a greater `color{red}(s)`-character is more electronegative and can hold the electron pair of `color{red}(C—X)` bond more tightly than `color{red}(sp^3)`-hybridised carbon in haloalkane with less `color{red}(s)`-chararcter.
● `color{red}(C—Cl)` bond length in haloalkane is `177` pm while in haloarene is `169` pm.
● Because it is difficult to break a shorter bond than a longer bond, therefore, haloarenes are less reactive than haloalkanes towards nucleophilic substitution reaction.
(iii) `color{green}("Instability of phenyl cation ")` : In case of haloarenes, the phenyl cation formed as a result of self-ionisation will not be stabilised by resonance and therefore, `color{red}(S_N 1)` mechanism is ruled out.
(iv) Because of the possible repulsion, it is less likely for the electron rich nucleophile to approach electron rich arenes.
(i) `color{green}("Resonance effect ")` : In haloarenes, the electron pairs on halogen atom are in conjugation with `color{red}(π)`-electrons of the ring and the resonating structures as shown in fig.1 are possible.
`=>` `color{red}(C—Cl)` bond acquires a partial double bond character due to resonance.
● Therefore, the bond cleavage in haloarene is difficult than haloalkane and therefore, they are less reactive towards nucleophilic substitution reaction.
(ii) `color{green}("Difference in hybridisation of carbon atom in C—X bond")` : In haloalkane, the carbon atom attached to halogen is `color{red}(sp^3)` hybridised while in case of haloarene, the carbon atom attached to halogen is `color{red}(sp^2)`-hybridised. See fig.2.
`=>` The `color{red}(sp^2)` hybridised carbon with a greater `color{red}(s)`-character is more electronegative and can hold the electron pair of `color{red}(C—X)` bond more tightly than `color{red}(sp^3)`-hybridised carbon in haloalkane with less `color{red}(s)`-chararcter.
● `color{red}(C—Cl)` bond length in haloalkane is `177` pm while in haloarene is `169` pm.
● Because it is difficult to break a shorter bond than a longer bond, therefore, haloarenes are less reactive than haloalkanes towards nucleophilic substitution reaction.
(iii) `color{green}("Instability of phenyl cation ")` : In case of haloarenes, the phenyl cation formed as a result of self-ionisation will not be stabilised by resonance and therefore, `color{red}(S_N 1)` mechanism is ruled out.
(iv) Because of the possible repulsion, it is less likely for the electron rich nucleophile to approach electron rich arenes.