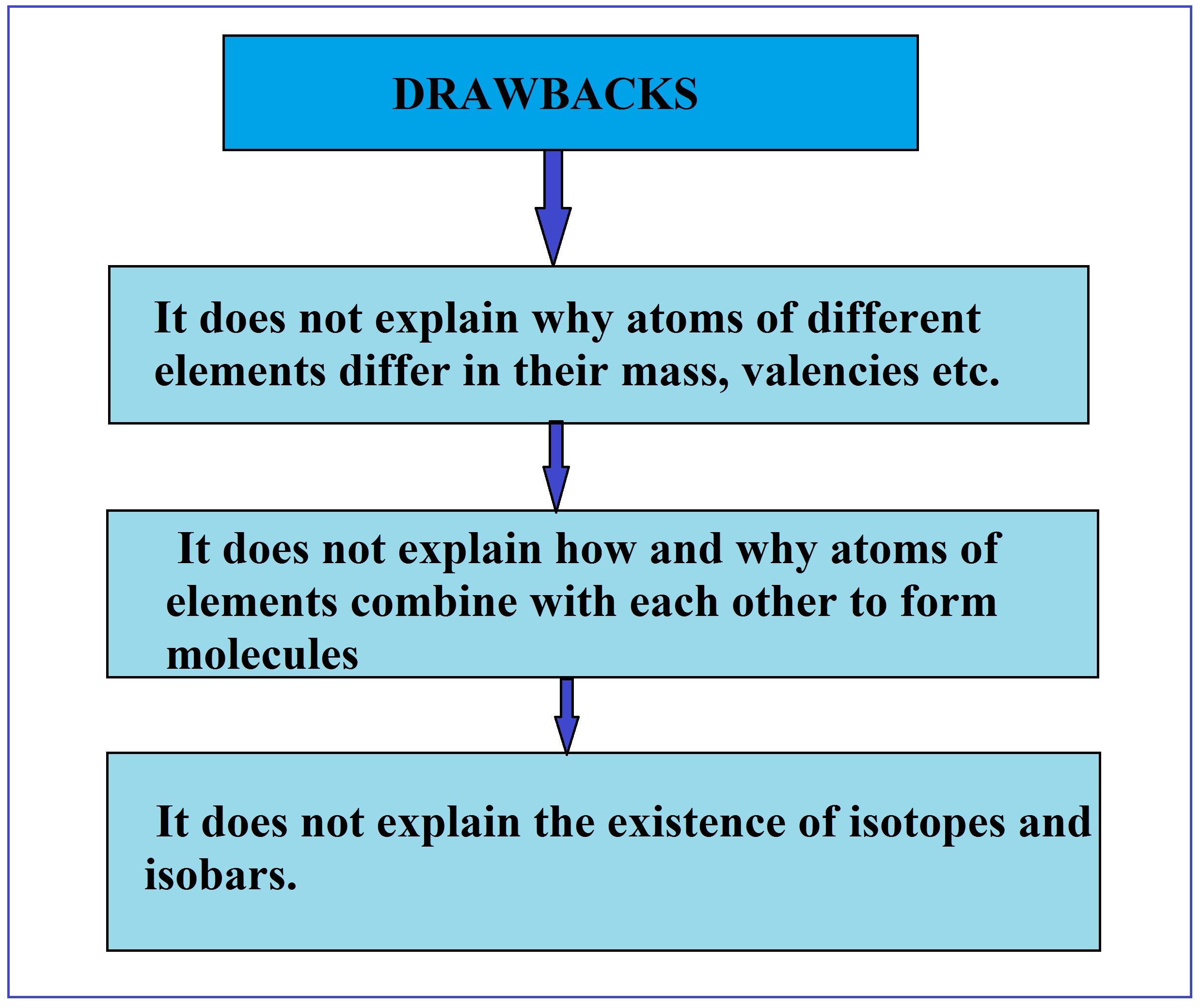
Dalton's Atomic Theory
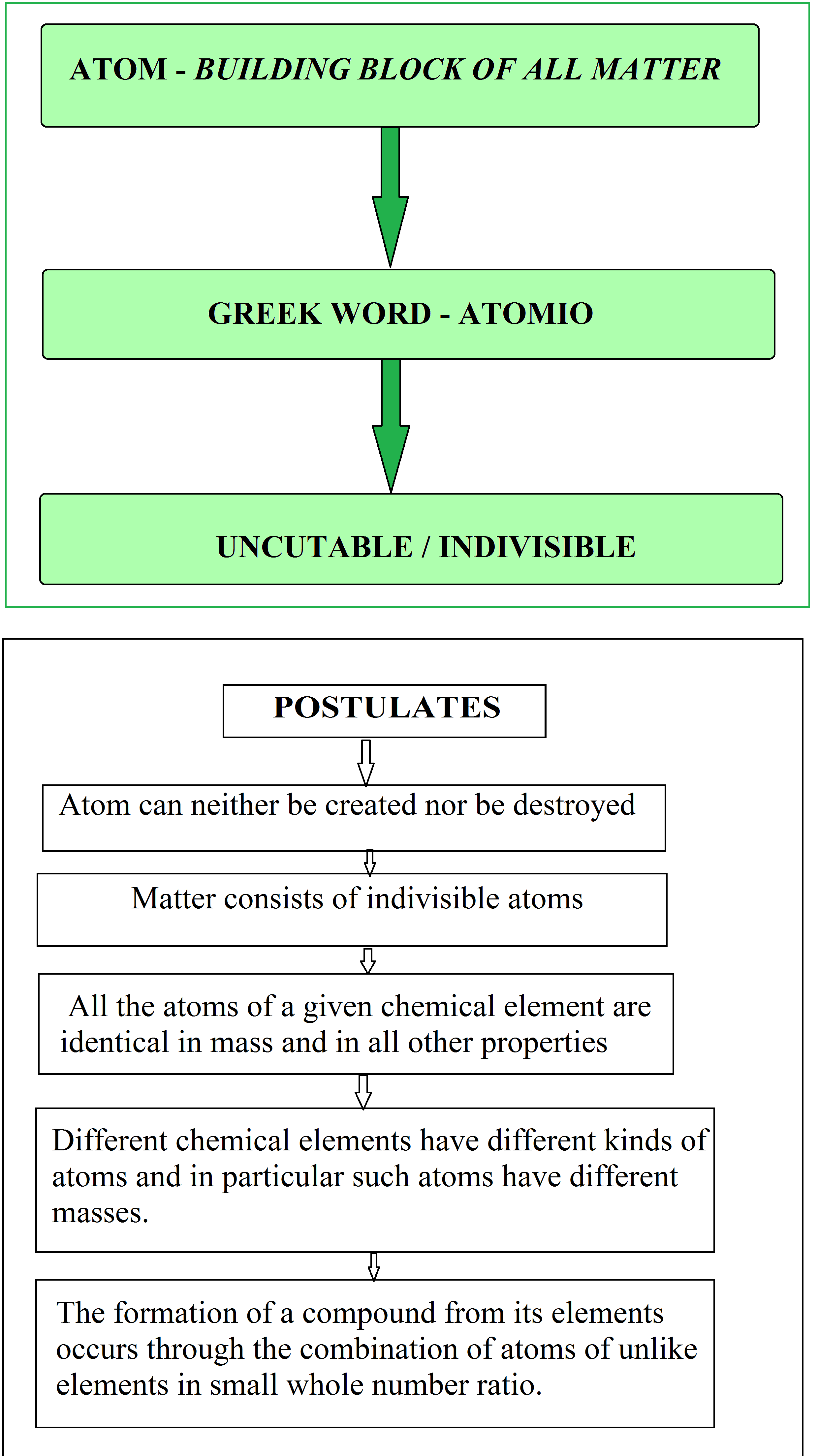
John Dalton (1890) put forward the first theory about the structure of the matter. Its main postulates are
`=>` Atom can neither be created nor be destroyed.
`=>` Matter consists of indivisible atoms.
`=>` All the atoms of a given chemical element are identical in mass and in all other properties.
`=>` Different chemical elements have different kinds of atoms and in particular such atoms have different masses.
`=>` The formation of a compound from its elements occurs through the combination of atoms of unlike elements in small whole number ratio.
`=>` Atom can neither be created nor be destroyed.
`=>` Matter consists of indivisible atoms.
`=>` All the atoms of a given chemical element are identical in mass and in all other properties.
`=>` Different chemical elements have different kinds of atoms and in particular such atoms have different masses.
`=>` The formation of a compound from its elements occurs through the combination of atoms of unlike elements in small whole number ratio.