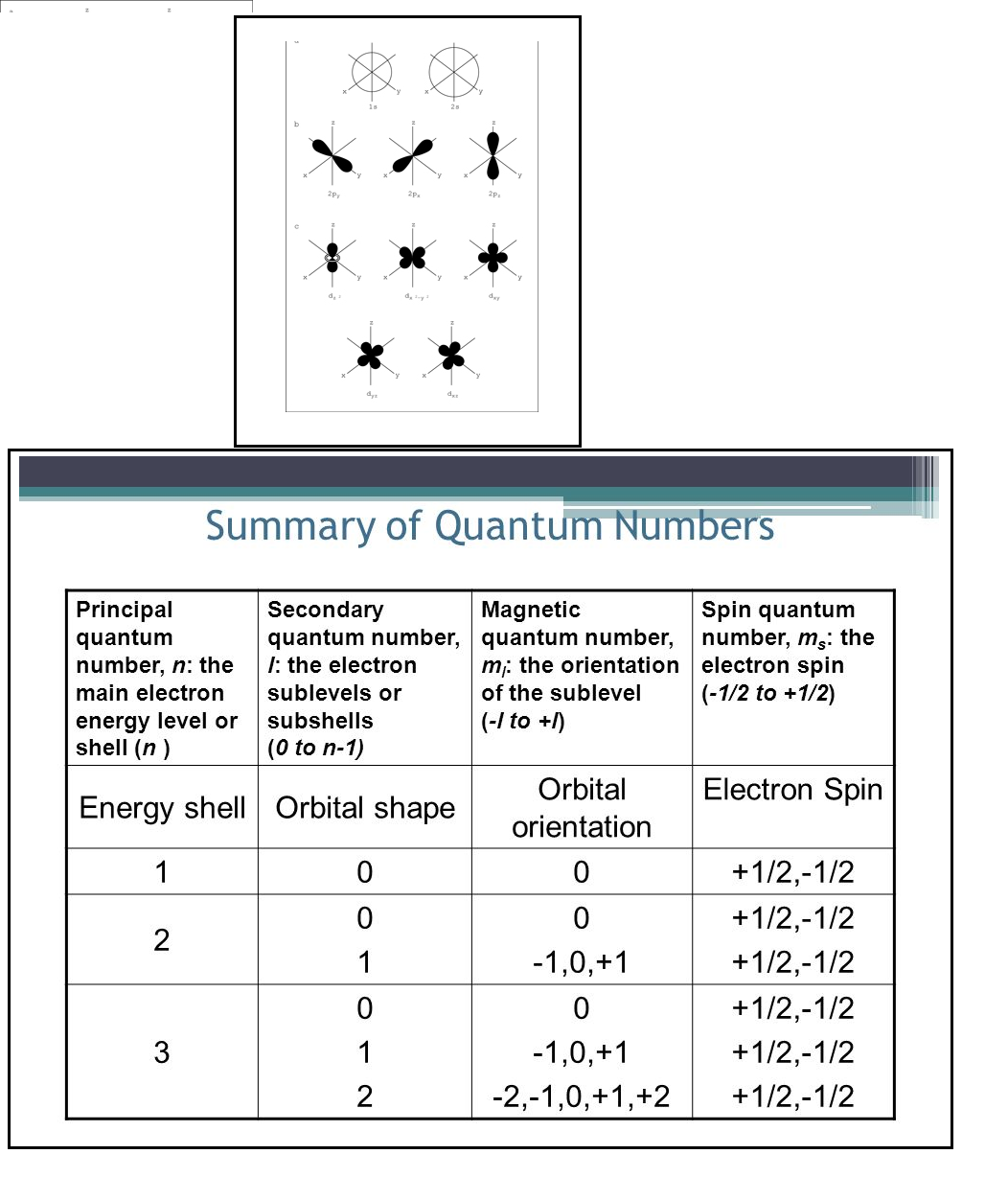
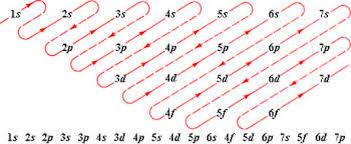
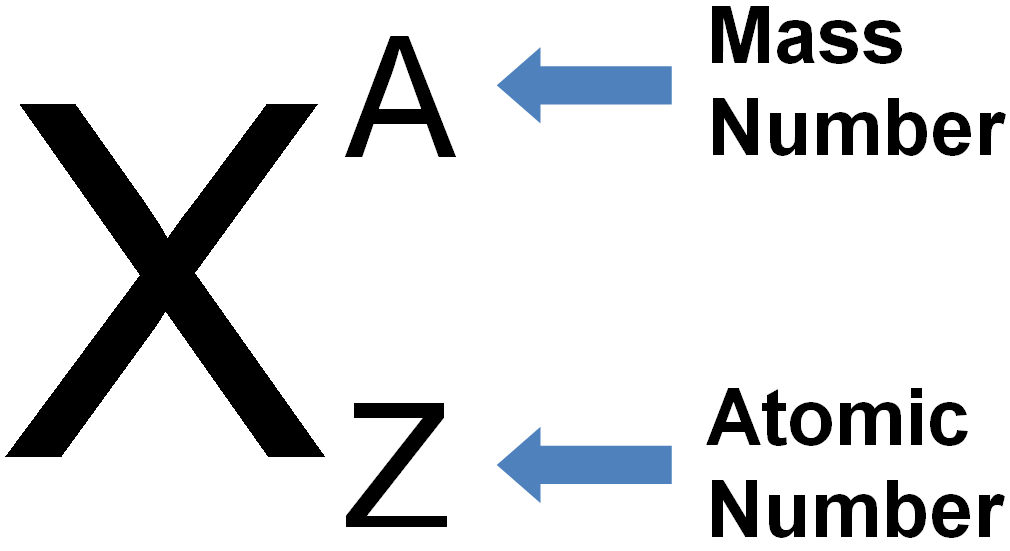`=>` According to `(n +l)` rule, the lower the value of `(n +l)` for an orbital the lower is its energy e.g. between `3d` and `4s`, the `4s (4 + 0 = 4)` be filled before `3d (3 + 2 = 5)`.
`=>` If two orbitals have same value of `(n + l)`, the orbital with lower value of `n` will be filled first e.g. between `2p` and `3s`, `2p (2+ 1 = 3)` will be filled first than `3s ( 3 + 0 = 3)`. The order of increasing energies is summed as `1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d,6p,7s,5f,6d,7p`
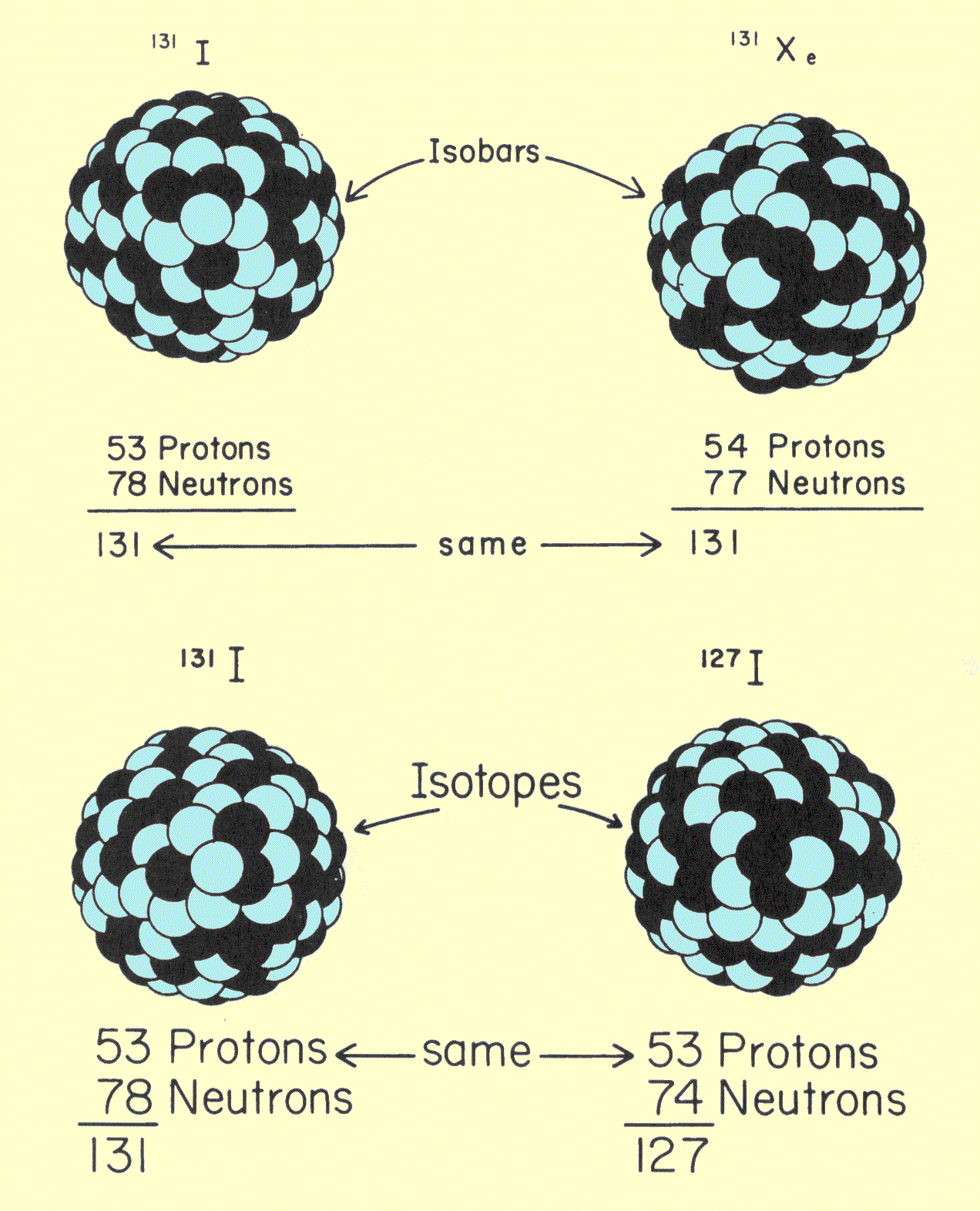
`text(Note) :` `text()_(24)Cr` and `text()_(29)Cu` do not obey this law.
`text()_(24)Cr = 1s^2 , 2s^2 , 2p^6 , 3s^2 , 3p^6 , 3d^5 , 4s^1`
`text()_(29)Cu = 1s^2 , 2s^2 , 2p^6 , 3s^2 , 3p^6 , 3d^(10) , 4s^1`.
`=>` The completely filled and half-filled sub-shells have lesser energy and thus assume more stability than any other arrangement. Thus `3d^5 , 4s^1` and `3d^(10) , 4s^1` are more stable arrangement than `3d^(4) , 4s^2` and `3d^9 , 4s^2` respectively.
`=>` According to `(n +l)` rule, the lower the value of `(n +l)` for an orbital the lower is its energy e.g. between `3d` and `4s`, the `4s (4 + 0 = 4)` be filled before `3d (3 + 2 = 5)`.
`=>` If two orbitals have same value of `(n + l)`, the orbital with lower value of `n` will be filled first e.g. between `2p` and `3s`, `2p (2+ 1 = 3)` will be filled first than `3s ( 3 + 0 = 3)`. The order of increasing energies is summed as `1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d,6p,7s,5f,6d,7p`
`text(Note) :` `text()_(24)Cr` and `text()_(29)Cu` do not obey this law.
`text()_(24)Cr = 1s^2 , 2s^2 , 2p^6 , 3s^2 , 3p^6 , 3d^5 , 4s^1`
`text()_(29)Cu = 1s^2 , 2s^2 , 2p^6 , 3s^2 , 3p^6 , 3d^(10) , 4s^1`.
`=>` The completely filled and half-filled sub-shells have lesser energy and thus assume more stability than any other arrangement. Thus `3d^5 , 4s^1` and `3d^(10) , 4s^1` are more stable arrangement than `3d^(4) , 4s^2` and `3d^9 , 4s^2` respectively.