Mendeleev's Periodic Table
D'mitri Mendeleev (also spelled as Mendeleef or Mandeleyev), a Russian chemist studied the properties of all the 63 elements known at that time and their compounds. Earlier attempts of classification were based on the similarities only in small units like a triad or in a small group. But the table given by Mendeleev represents the relationship in tverical columns and in horizontal rows. According to Mendeleev on arranging the elements in the increasing order of atomic masses, it was observed that the elements with similar properties repeat periodically.
In 1869, Mendeleev stated his observation in the form of the following statement which is known as the Mendeleev's Periodic Law.
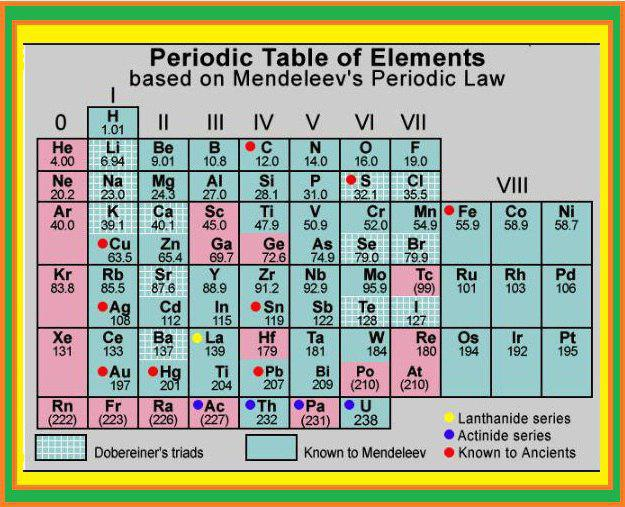
The chemical and physical properties of elements are periodic function of their atomic masses. Mendeleev arranged the elements in the form of a table which is known as the Mendeleev's Periodic Table as below :
`=>` Elements were arranged in increasing order of their atomic masses in horizontal rows till element whose properties were similar to those of the first element was came across.
`=>` This element was placed below the first element and thus started the second row of elements.
`=>` All the known elements were arranged in the periodic table proceeding in this way.
`=>` Gaps were left for possible elements still to be discovered. He predicted the properties of the elements that had not been discovered. He named these missing elements as Eka boron, Eka aluminium, Eka silicon. Later on, these elements were discovered and named as scandium, gallium and germanium, respectively
In 1869, Mendeleev stated his observation in the form of the following statement which is known as the Mendeleev's Periodic Law.
The chemical and physical properties of elements are periodic function of their atomic masses. Mendeleev arranged the elements in the form of a table which is known as the Mendeleev's Periodic Table as below :
`=>` Elements were arranged in increasing order of their atomic masses in horizontal rows till element whose properties were similar to those of the first element was came across.
`=>` This element was placed below the first element and thus started the second row of elements.
`=>` All the known elements were arranged in the periodic table proceeding in this way.
`=>` Gaps were left for possible elements still to be discovered. He predicted the properties of the elements that had not been discovered. He named these missing elements as Eka boron, Eka aluminium, Eka silicon. Later on, these elements were discovered and named as scandium, gallium and germanium, respectively